
The trends need to know are as follows:
1. Metallic properties
2. Atomic Radius
3. Ionization energy
4. Electronegativity
5. Reactivity
6. Ion Charge
7. Melting/Boiling Point
8. Density
1. Metallic properties
2. Atomic Radius
3. Ionization energy
4. Electronegativity
5. Reactivity
6. Ion Charge
7. Melting/Boiling Point
8. Density
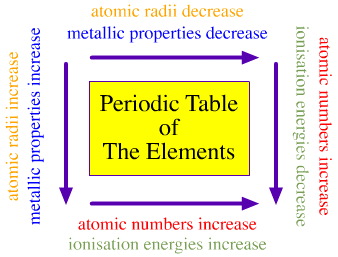
If you were to look carefully at many of the properties of the elements, you would notice something besides the similarity of the properties within the groups. You would notice that many of these properties change in a fairly regular fashion that is dependent on the position of the element in the periodic table. As you compare elements from left to right across the periodic table, you will notice a trend or regular change in a number of properties. The same thing happens if you go up and down on the periodic table and compare the properties of the elements.
Atomic Radii

1) As you move down a group, atomic radius increases.
| WHY? - The number of energy levels increases as you move down a group as the number of electrons increases. Each subsequent energy level is further from the nucleus than the last. Therefore, the atomic radius increases as the group and energy levels increase. | ![[Image]](http://www.chem.tamu.edu/class/majors/tutorialnotefiles/Atomicradii.gif) |
2) As you move across a period, atomic radius decreases.
- WHY? - As you go across a period, electrons are added to the same energy level. At the same time, protons are being added to the nucleus. The concentration of more protons in the nucleus creates a "higher effective nuclear charge." In other words, there is a stronger force of attraction pulling the electrons closer to the nucleus resulting in a smaller atomic radius.

Definition: The energy required to remove the outermost (highest energy) electron from a neutral atom in its ground state.1) As you move down a group, first ionization energy decreases.WHY?
2) As you move across a period, first ionization energy increases.Electrons are further from the nucleus and thus easier to remove the outermost one. "SHIELDING" - Inner electrons at lower energy levels essentially block the protons' force of attraction toward the nucleus. It therefore becomes easier to remove the outer electron
WHY? - As you move across a period, the atomic radius decreases, that is, the atom is smaller. The outer electrons are closer to the nucleus and more strongly attracted to the center. Therefore, it becomes more difficult to remove the outermost electron.Exceptions to First Ionization Energy Trends
1) Xs2 > Xp1 e.g. 4Be > 5B
| ![[Image]](http://www.chem.tamu.edu/class/majors/tutorialnotefiles/IEexceptions.gif) |
MetalsCommon characteristics:
NonmetalsCommon characteristics:
The differences in the characteristics of metals and nonmetals can be explained by the following:
1) As you move across a period, metallic character decreases and nonmetallic character increases.2) As you move down a group, metallic character increases and nonmetallic character decreases.Semimetals (Metalloids)
B | Si | Ge | As | Sb | Te | Po | At |
Metals and non-metals show different
trends.
The most reactive metal is Francium; the
most reactive non-metal is Fluorine
Ion Charge
elements ion charges depend on their
group (column).
Melting Point
Elements in the center of the table of the
highest melting point
Noble gases have the lowest melting
points
Starting from the left and moving right,
melting point increases (until the middle of
the table)
DENSITY
metals will tend to have higher density because of their positive charge. And radioactive elements also are also very dense. But you can say that as you go down the table, atoms are more dense.

Trend of Ionization:http://www.youtube.com/watch?v=ywqg9PorTAw
General Trend:http://www.youtube.com/watch?v=CHHy2ex0dw4
http://www.youtube.com/watch?v=h7XWqwgZII0&feature=related
No comments:
Post a Comment