Oh no, are those the same BOHR-ing Bohr diagrams again?!

Nope! These are all-new, EXCITING Chem 11 diagrams that you've never heard of before! To start off, we have the electron dot diagram!
Electron Dot Diagrams
For these diagrams, there are a couple of things you need to know:
- the nucleus, aka the centre of the diagram, is the atomic symbol of the element you are drawing
- bonds can be drawn on all four sides of the "nucleus" or atomic symbol
- each single dot represents one bond
- each side must have at least one bond/"dot" before you can add on a second one
- valence electrons are shown

Lucky Number Eight:
One thing you have to remember when drawing electron dot diagrams is the Octet Eight rule: every single element MUST have eight dots surrounding its nucleus. WHY? Because in order to have a stable valence shell like the noble gases, it must fill its outermost shell with eight electrons!
The ONE exception is Hydrogen, which is happy with just two "dots".
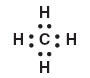
Notice how the Carbon has eight electrons, while each Hydrogen has two. Everyone is happy, because they are sharing, yay.
Lewis Structure Diagrams
In Lewis structure diagrams, Hydrogen and Fluorine never go into the centre. The metal goes into the centre, or the element which requires the most valence electrons to gain stability.
Adding and Subtracting:
For Lewis diagrams, add up the total number of valence shell electrons, THEN add or subtract the charges on the compound to find out how many "dots" there should be in your diagram.
For example, for NO3, the charge is -1. N has 5 valence electrons, O has 6. 5 + 6 + 1 (for the added electron that caused the negative 1 charge) is 12. There should be 12 dots in the diagram.
In ionic compounds, draw a big [ ] around the entire structure, and add the charge on the top right-hand corner, like so:

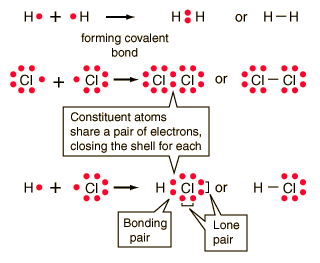
In covalent compounds, use lines to indicate bonds.
No comments:
Post a Comment