Alkenes and Alkynes
- Carbon can form double and triple bonds with Carbon atoms.
- When multiple bonds form, fewer hydrogens are attached to the Carbon atom.
- The position of the double/triple bonds always has the lowest number and is put in front of the parent chain.
Alkenes
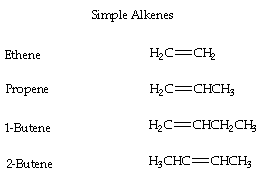
- Alkenes are hydrocarbons with one or more double bonds located between carbon atoms leading to an "unsaturated" hydrocarbon.
- The ending is changed from -ane (alkane) to -ene for alkenes
- ex. CH2 = CH2 equals to ethene
- With Alkenes, some molecules have the same structure but different geometry. This is called Geometric Isomers.
- If two adjacent carbons are bonded by a double bond AND have side chains on them two possible compounds are possible = Cis or Trans.
- Cis: If the larger groups are both above or below the plane of the bond.
- Trans: If the larger groups are across the plane of the bond.
- If there are two identical groups on either end of the double bond, there is no need for cis or trans.
Alkynes
- Alkynes are hydrocarbons with one or more triple bonds located between carbon atoms leading to an "unsaturated" hydrocarbon.
- The ending is changed from -ane (alkane) to -yne for alkenes
- ex. CH =(add a dash to the equal sign for the triple bond aka three lines) CH equals to ethyne
Now here are some images:












Videos:
1) http://www.youtube.com/watch?v=l6m7zOto9oU
2) http://www.youtube.com/watch?v=KWv5PaoHwPA&feature=related
3) http://www.youtube.com/watch?v=6NygjuEFkIc
4) http://www.youtube.com/watch?v=3cfVBC2pS38&feature=relmfu
5) http://www.youtube.com/watch?v=jF46f8vkJPg&feature=related
6) http://www.youtube.com/watch?v=ryTCHJIi6d8&feature=related
Worksheets:
1) http://www.gahrhs.org/ourpages/auto/2010/5/28/39679088/Alkene%20Alkyne%20worksheet.pdf
2) http://www.pkwy.k12.mo.us/west/teachers/anderson/pack10/WS107.pdf
3) http://fcw.needham.k12.ma.us/~Janet_Fasano/FOV1-00108B66/FOV1-0010CD86/Alkene%20and%20Alkyne%20Worksheet.pdf
4) http://ewalerko.net/grs0025t.pdf
Well that is it.... for now!! Have a great day everyone! :)






 (negative) charge.
(negative) charge. (positive) charge.
(positive) charge.



 Ionic Bonding
Ionic Bonding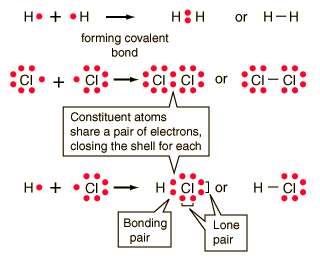
 Polar Covalent Bonding
Polar Covalent Bonding Polar Covalent Bonding
Polar Covalent Bonding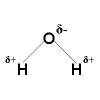 Polar Covalent Bonding
Polar Covalent Bonding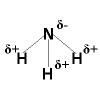 Polar Covalent Bonding
Polar Covalent Bonding Polar Covalent Bonding
Polar Covalent Bonding Ionic Bonding
Ionic Bonding Ionic Bonding
Ionic Bonding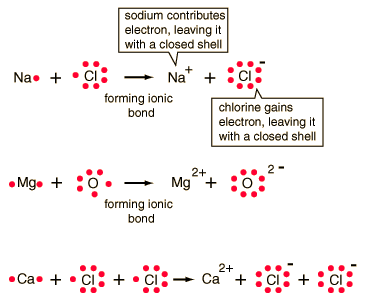 NOTE: Shows Valance Electrons!
NOTE: Shows Valance Electrons! Sodium Chloride Crystal Structure
Sodium Chloride Crystal Structure




![[Image]](http://www.chem.tamu.edu/class/majors/tutorialnotefiles/Atomicradii.gif)

![[Image]](http://www.chem.tamu.edu/class/majors/tutorialnotefiles/IEexceptions.gif)
