Now, Organic chemistry is simply the chemistry of carbon?...not quite....because such compounds like CO2, CO, CN are NOT organic! So Why do they say Organic Chemistry is the chemistry of carbon?
Well, it is actually the chemistry of hydrocarbons= carbons and hydrogens. And the end products are amazing! They examplify our everyday products such as clothing, plastic, alcohol and much more...
But those two are just the basic requirement of organic compounds, we can also add Oxygen, Nitrogen, etc. to elaborate...oops..seems like I have spilled the next topics...>. >''
Special properties exclusively to Organic Compounds are:
1. low melting points
2. weak/ non-electrolytes
3. from chains of carbon atoms linked in:
a. straight line

b. circular pattern

c. branched pattern

4. can be linked in single bond, double bond, and triple bond.
single bonds are called alkane, double bonds are called alkene, triple bonds are called alkyne.
Properties of Alkanes:
1. Saturated hydrocarbons with single bonds!
2. compounds usually end with -ane
3. represented with structural, condensed, molecular and ball-stick model (rarely used)
* Abbreviated formula = condensed formula

Here is a table of three different formulas.
Have you noticed a pattern between the Carbon molecules and Hydrogen molecules?
...If you didn't I will show you now "C n H 2n+2"

Alkanes can be branched or subsitutited with Alkyl groups which is an alkane having lost one Hydrogen atom. The alkyl groups end in "-yl":


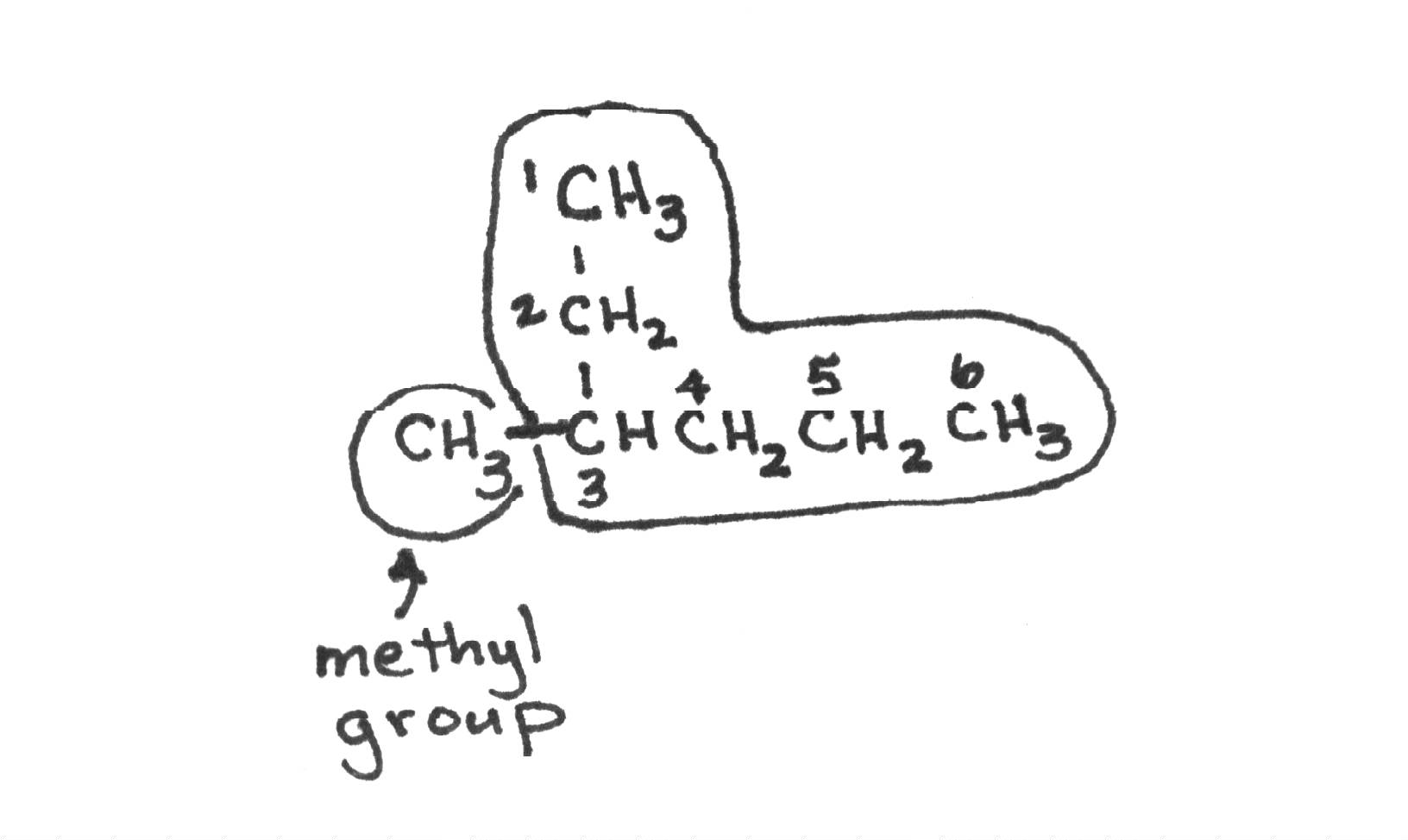
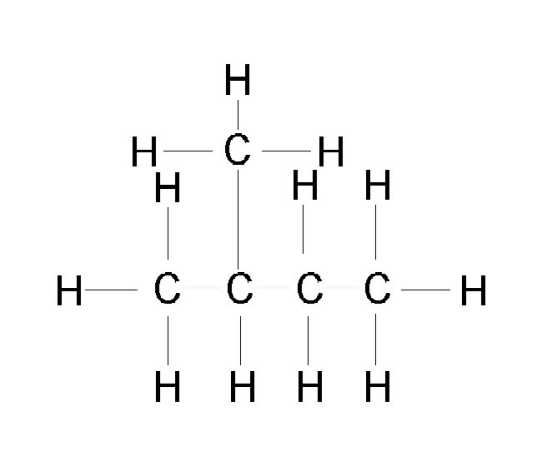 |
| This is an example of a branched hydrocarbon named 2-methylbutane Naming is the toughest part, but also the neatest of Organic Chemistry, so pay attention! Rules for Alkane Nomenclature (IUPAC): 1. consider LONGEST CONTINUOUS CHAIN 2. Name the alkyl groups attached 3. give carbon number where the alkyl group has the lowest carbon number 4. put Carbon#(if repeated, then put twice and separate with a "comma")-alkyl name (if repeated use prefixes to indicate: Dimethyl, tetraethyl,...)the longest chain name. |
http://www.5min.com/Video/Learn-about-Organic-Chemistry-3-111429856
ORGANIC CHEMISTRY 3
There is a series of tutorial ongoing for Organic Chemistry, so you will get the full picture!
http://www.5min.com/Video/Learn-about-Organic-Chemistry-4-111430078
ORGANIC CHEMISTRY 4
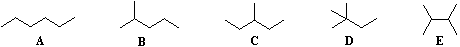
No comments:
Post a Comment