Finding Out More About Matter
(source: Heath Chemistry Textbook, pages 25-34, 36-39)
As a chemist, you need to develop the ability to make detailed observations and careful analyses about the world around you. This is especially true when studying a subject such as matter. Some questions that might be raised during your course of studies are:
- How do certain types of matter differ from one another?
- How is matter changed from one form to another?
- Can matter be broken down into further states of separation?
2-1 What You Know about Matter
Matter such as water comes in many different forms. It can be frozen as a solid, it can exist in liquid form, and it can be heated up and turned into a vaporous gas.
Water from different sources can look and taste differently, and have different boiling points. A
boiling point is the temperature at which a liquid turns into gas. All of these (colour, taste, and boiling point) are characteristics scientists use when identifying matter.
2-2 Purifying Matter

As a child, have you ever went outside after it rained and scooped out a bucketful of muddy water? Well, you might have noticed that after a few days, the dirt will have sunk down to the bottom, while the water still stayed at the top. This separation proves that the muddy water is a
mixture - two or more kinds of matter that have their own identities mixed together.
Deciding when a substance is pure (one kind of matter only), or when it's a mixture can be difficult. Some methods used to find the difference between the two are:
- Using a flashlight: the light shone on a settled mixture will be scattered; the light shone on some pure substances such as tap water will not scatter
- Adding alum and lime: this creates a sticky substance that will attract all the particles in the water, leaving only pure water behind
- Distillation: because of different boiling points, mixtures can be heated until they separate

Substances such as sugar or salt will dissolve in water. When you shine a light on them, the light doesn't scatter. Salt water or sugar water mixtures are called
solutions.
A substance is only considered pure if there seems to be no possible way to break it down any further.
2-3 Characteristics of Pure Substances

Another way to distinguish between mixtures and pure substances is by their boiling points. As a general rule, the boiling point of mixtures gradually increase as time goes on, while the boiling point of pure substances stay the same.
There are, of course, exceptions to the rule, such as a mixture of water and grain alcohol, which can't be separated by distallation.
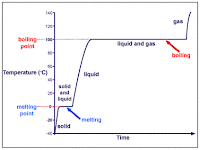
The temperature at which a liquid changes to a solid is called the freezing point. The freezing point is the same as the melting point, when a solid turns into a liquid.
2-4 Chemical and Physical Changes

As had been covered in the previous posts, a
chemical change is a change that sees new products with different properties being formed. A
decomposition is a chemical change in which a substance decomposes to produce something new.
Physical changes are changes that are reversible and do not see new products being formed. If the change yields a substance that looks the same as the original, smells the same, weighs the same, and melts at the same temprature, chances are, it's a physical change.
2-5 Compounds and Elements
The difference between decompostion and distillation is that, since decomposition shows a process in which new products are formed, it's a chemical change.
Distillation merely separates two substances that are already mixed together, hence why it's a physical change.
 Compounds
Compounds are pure substances that can be broken down further. For example, water is made up of hydrogen and oxygen molecules. Substances such as hydrogen and oxygen can't be decomposed. They are part of a group of elements which serve as the building blocks for all other kinds of matter.
states that while mixtures can have any possible composition, the composition of compounds always remain the same.
The law of multiple properties states that different compounds can be made with differing amounts of the same elements.
Matter is Made of Atoms
Macroscopic observations are observations you can make with the bare senses. Some observations are smelling, seeing, and tasting. However, these rough observations can only help you so much. How can you explain what you have witnessed?
2-7 Atoms
If you take a sheet of paper and rip it up into smaller and smaller pieces, sooner or later, you'll come to the smallest piece that still carries the characteristics of the original piece of paper. That smallest piece is called the
atom.
Since we can't see atoms with our naked eyes, we use coloured spheres to represent them.
2-8 Elements
Elements are pure substances that can't be broken down any further. Therefore, it can be concluded that each element has only one, unique type of atom. The
atomic number is the number assigned to each such type of atom.
 When a substance is in a solid form
When a substance is in a solid form, the atoms that make up the substance are stuck together tightly. They don't have much room for movement, and can only vibrate at the most.
When a substance is in a liquid form, the atoms slide around more easily, but gravity still makes liquids take to the shape of their container.
When a substance is heated up, the atoms start moving past each other quicker and with more force. Eventually, they may break out altogether, turning the liquid into a gas.
Molecules are particles made up of more than one atom.
Atoms explain the different boiling points of substances. More energy is required for larger atoms to "break loose", so therefore substances with larger atoms have higher boiling points.
2-9 Compounds
Compounds can be separated further into its individual elements by using either heat or electricity. These two forms of separation provide the energy needed to break apart molecules and atoms.
Take water, for example. When water in its solid form melts, the molecules come apart from each other. The more it's heated, the further the particles break down. When water is heated into a gas, not only do the molecules break apart from each other, but they separate further into hydrogen and oxygen atoms, creating new substances.
Ions are electrically charged particles. Compounds that produce ions conduct electricity, and those that don't produce ions do not conduct electricity.