The lab we did focused on the heating and cooling process of a dodecanoic acid.
- Here is a quick intro to melting and coolng points:
Every pure substance has a melting point, a characteristic tempature at which it melts. This property of individual melting points can be used by chemists to identify substances. Likewise, when a liquid pure substance cools, it freezes at a characteristic tempature. Freezing points can therefore be used to differentiate between methanol and water, for example. Both of these are colorless liquids, but methanol freezes at -94 degrees celsius, whereas water freezes at 0 degrees celsius.
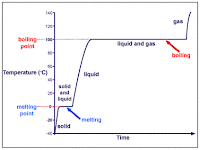
Here are some images that also explain melting and freezing points:
Now for a quick outline of the lab:
- Equipment;
ring stand, buret clamp, hot plate, test tube which had the dodecanoic acid in it, beaker, 2 thermometers, and safety goggles. (lab apron optional)
- Procedure(s);
There were two procedures for this lab, one the heating process next the cooling process. We did the cooling process first which was alot faster and easier. The procedures were......

- Experimental Results;
We had one table to record our results and observations (down below) and after we completed that we created a graph to show the heating curve and cooling curve of our acid. But we did not have time to finish our graph so we will complete it next class.
- Follow-Up and Questions;
Since I already mentioned above we did not get to completely finish our lab. We did more than half: we finished the experiement, the table, and most of the report write up but just have to finish these questions and the graph.
1. How would you exaplain the plaeaus in your heating and cooling curve?
2. Suppose the more dodecanoic acid had been used in Part 1. What would be the change in appearance of the new cooling curve? Sketch it.
BUT here is essentialy what our graph will look like....
(Left) Heating Curve & Cooling Curve (Right)
EXTRA:
- Video;
1. This video shows a science do a experiment similiar to what we did:
http://www.youtube.com/watch?v=gPY2K5RGZQM
- Last but definetely not least:
Jokes to make you chuckle;
1) Q: What is the dullest element?
A: Bohrium.
2) Q: Why are chemists great for solving problems?
A: They have all the solutions.
3) Q: What is the difference between Chemistry and cooking?
A: In chemistry, you should never lick the spoon.



THANK YOU AND HAVE A GREAT THANKSGIVING !!!!!
EXTRA:
- Video;
1. This video shows a science do a experiment similiar to what we did:
http://www.youtube.com/watch?v=gPY2K5RGZQM
- Last but definetely not least:
Jokes to make you chuckle;
1) Q: What is the dullest element?
A: Bohrium.
2) Q: Why are chemists great for solving problems?
A: They have all the solutions.
3) Q: What is the difference between Chemistry and cooking?
A: In chemistry, you should never lick the spoon.



THANK YOU AND HAVE A GREAT THANKSGIVING !!!!!






No comments:
Post a Comment