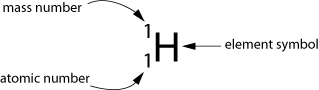
Families. We all have them. Sometimes, they may drive us crazy. But love them or hate them, we're stuck with the ones we've got, so we have to stick with them for what they're worth.
Just like us, the periodic table can be grouped into certain families as well! These families are:

Alkali metals: Shown as the yellow strip on the far left, alkali metals display properties such as high density levels, a single valence electron, low ionization energies, and a large atomic radii.
Alkali earth metals: Shown as the bright, bright blue strip next to the alkali metals, alkali earth metals have two electrons in their outer shells, low electronegativities, and slightly smaller atomic radii than the alkali metals.
Transition metals: These metals form the huge block between the alkali earth metals and the metalloids. They have high melting and boiling points, are malleable, and are good conductors of electricity.
Metalloids: These form a staircase consisting of boron, silicon, germanium, arsenic, antimony, and tellurium. Polonium is still under debate regarding its status as a metalloid. Metalloid properties vary widely. They have differing boiling and melting points, and are good semi-conductors.
Halogens: Halogens, as shown by the strip of pale yellow, are highly reactive elements. They display high levels of electronegativity, and all have seven valence electrons.
Noble gases: The orange family on the far right, noble gases tend to be very stable because they have full outer shells. Noble gases have low boiling points and rarely lose or gain electrons. They display low electronegativity.
Rare earths: These are the two sky blue rows at the very bottom, the Lanthanide series and the Actinide series. These metals are good conductors, are usually silvery in colour, and have high densities.
And now here's a nice poem by Michael Carungi!
Carbon, the Champion
Carbon is the element that is the basis
Of all organic life in all places
It can bond with elements, that's a fact
Over ten million compounds to be exact
When united with a substance like air
Carbon dioxide is created, which is used to prepare
The growth of plants. And when Carbon is combined
With Hydrogen, fuels are made. Diamonds and Graphite are a kind
Of Carbon, where Diamonds are the hardest ever known
And a softer substance than Graphite is unknown
Carbon also has the highest melting grade
And Carbon cannot be artificially made.
Yes, Carbon is truly a great thing
And it should be declared the elemental king.
UNTIL NEXT TIME!




 or the Russian Dmitri Mendeleev. Both chemists produced remarkably similar results at the same time working independently of one another. This consisted of about half of the known elements listed in order of their atomic weight and demonstrated periodic valence changes as a function of atomic weight. Unfortunately for Meyer, Mendeleev's table became available to the scientific community via publication (1869) before Meyer's appeared (1870)!
or the Russian Dmitri Mendeleev. Both chemists produced remarkably similar results at the same time working independently of one another. This consisted of about half of the known elements listed in order of their atomic weight and demonstrated periodic valence changes as a function of atomic weight. Unfortunately for Meyer, Mendeleev's table became available to the scientific community via publication (1869) before Meyer's appeared (1870)! 


 (we use the E)
(we use the E)