|We are starting a new unit today called the Atomic Theory.|
|This is the start of atom and where ideas of atoms emerged...|
|Why do you think ancient philosophers were so interested in the phenomenon of atoms? |
|Perhaps they were curious about the foundation of all matters and that was when they came up with atom...|
- Aristotle became the first to propose that all matter consists of four elements either
|EARTH| |WATER| |FIRE| OR |AIR|
 Aristotle: http://www.iep.utm.edu/aristotl/
Aristotle: http://www.iep.utm.edu/aristotl/
 Aristotle: http://www.iep.utm.edu/aristotl/
Aristotle: http://www.iep.utm.edu/aristotl/- Alchemist Brought Au to Earth by changing the mechanics of metal!
- The Four Element theory lasted for about 2000 years!
- Democritus proposed that atom = indivisible particles though this was not a testable theory and merely a model.=[ There was also no discovery of nucleus, proton or electrons. Thus, it cannot be used to explain chemical reactions
 Alchemist full metal image
Alchemist full metal image- Lavoisier started 1st version of Law of Conservation of Mass & Law of Definite Proportions.
Law of Definite Proportion means that the % of an element on a compound will never change!
- Proust proposed: even if compound are broken down, the ratio in the compound would still apply to the products.
- Dalton provided a vision of an atom = solid, sphere and his experiments and discoveries were based on the Law of Conservation of Mass
- Dalton's Law #1: Elements made up of atoms
- Dalton's Law #2: All atoms of a element are identical.
- Dalton's Law #3: atoms are distinguished by relative weight
- Dalton's Law #4: Atom(of different element) + Atom(of different element) = chemical Compound, with same # of same types of atom
- Dalton's Law #5: Atoms are indivisible, non-destructable and they cannot be created.
Thomson gave new characteristics to an atom: they are solid spheres with negative particles embedded (electrons) and positive particles (protons). He figured out the mass of electron, 9.10938188 x 10 -28 grams.

Adding to Thomson's discovery, RUtherford explained that all the positive particles gathers in the dense centre of an atom. They electrons are outside of the dense positive center. His model is called the planetary model.
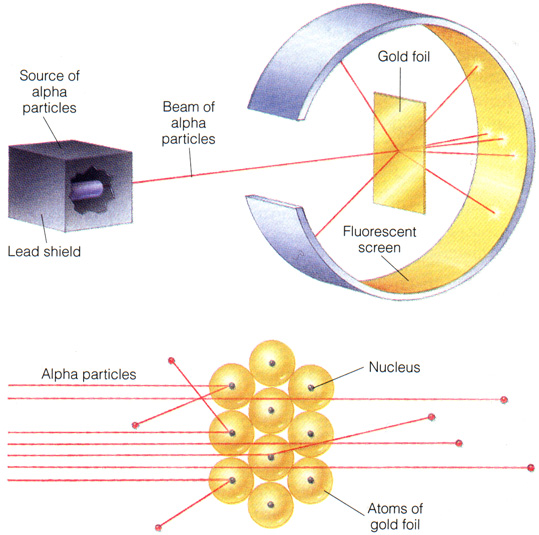
this is the famous GOLD LEAF experiment provides evidence for the positive centre of an atom.
Niels Bohr contributed to this RACE of ATOM most notably for discovering the Electrons in specific energy level or shells outside the nucleus.
 |
 BOHR diagram of atoms |
particle of an element that reatains the properties of element. There are 3 kinds of particles called subatomic particles: e, p, n
http://www.youtube.com/watch?v=h0UllT0DE7s&feature=related
This is an exellent video presenting the historical timeline of the atomic theory!
ENJOY~
No comments:
Post a Comment