Atomic Structure!
- The subatomic particles are: protons, neutrons and electrons.
˜Proton:
Symbol =
 (we use the P)
(we use the P)Relative Mass = 1
Electric Charge = +1
Location in the Atom = nucleus
˜Neutron:
Symbol = Relative Mass = slightly bigger than 1
Electric Charge = 0
Location in the Atom = nucleus
˜Electron:
Symbol =  (we use the E)
(we use the E)Relative Mass = 0
Electric Charge = -1
Location in the Atom = cloud surronding the nucleus
- In a Neutral atom, # of Protons = # of Electrons
Atomic Number (Z): The proton number
- The Atomic Number (Z) is the number of protons found in the nucleus of an atom.
- Atoms have no overall electric charge (i.e. charge of atom is zero).
- Atomic Number = # of protons = # of electrons
Ions:
- Atoms that have gained or lost electrons are called ions.
- An ion is an electrically charged atom (or groups of atoms).
- Negatively-charged ion = anion
- Positively-charged ion = cation
- For Ions; # of electrons = protons - charge
Mass Number (A):
- Is the total number of protons and neutrons or atomic mass number.
- Atomic Number = the # of protons
- Atomic Mass = # of protons + # of neutrons
- Number of neutrons = Mass Number - Atomic Number
- Mass Number = # of protons + # of neutrons
Atomic Mass:
- The average mass of an element's isotopes.
- The atomic mass is very close (not exact!) to the mass number.
- # of neutrons = Mass Number - Atomic Number
Atomic Mass - Atomic Number
- The atomic mass is an average! The mass number is usually calculated by rounding the atomic mass to the nearest whole number.
Isotopes:
- Are atomic species having the same atomic number (protons) but different atomic masses/mass numbers (neutrons). Basically, same # of protons and electrons, but different # of neutrons.
Whew! Alot of information but pretty simple to comprehend, which is a very good thing :) So now let's give you some important visuals!





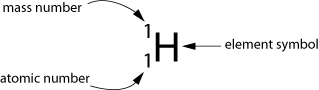



Hope those are helpful, and now here are some worksheets & extra information for you:
1) http://www.sciencejoywagon.com/chemzone/02atomic-structure/
2) http://misterguch.brinkster.net/propertyworksheets.html
3) http://docs.google.com/viewer?a=v&q=cache:Svl1HS3qzfsJ:misterguch.brinkster.net/001_021.doc+atomic+structure+worksheet&hl=en&gl=ca&pid=bl&srcid=ADGEESj_WYrtiEN2lX55CTrVQlm1hc7YmKY1mVbgw1DoKBPwfd7ZkvmnBtHXM9MonGdgMHr7rYPbP_zspbkgYZtxil2dk5ld9UqPcFm79Mukr5YOnQD-v3EXpsaki74QhGzie3QH_Cry&sig=AHIEtbTL6KdKPbx4-wFOQzdx-a1ZI3Et9g&pli=1
4) http://cmsweb1.loudoun.k12.va.us/52820831134912597/lib/52820831134912597/Atoms%20and%20Atomic%20Theory/Homework/ws.atomic.20structure.20set.pdf
5) http://www.allaboutcircuits.com/worksheets/atomic.html
6) http://staff.fcps.net/jswango/unit2/atomic_structure/Basic%20Atomic%20Structure%20Worksheet.pdf
7) http://chemistry.about.com/library/weekly/blatomquiz2.htm
8) http://www.softschools.com/quiz_time/chemistry/atomic_structure/theme81.html
Last but not least, here are some youtube videos:
1) http://www.youtube.com/watch?v=7ohfJ9ku8gc&feature=fvwrel
2) http://www.youtube.com/watch?v=WWxnZK_g5ug
3) http://www.youtube.com/watch?v=Z6Y4Ffod1hQ&feature=related
Thanks for reading & have a great long weekend! Wooooooooooo!!! :)
No comments:
Post a Comment