Today in class we took notes of the....... Molar Volume of a Gas at STP
- Description of a STP: The definition is that STP is a standard condition that compares volume of gases. STP stands for Standard Temperature & Pressure. A STP is one atmosphere of pressure and a temperature of 0 degrees celsius and 273.1 K. At STP one mole of gas = 22.4 L.
- The two CONVERSION FACTORS:
1. 22.4L of gas 2. 1 mole of gas
1 mole of gas 22.4L of gas
Here is an example for you to practice:
1) calculate the volume occupied by 3.4g of ammonia at STP
= molar mass of ammonia (NH3) = 17 g/mole
= 3.4g x 1 mole = 0.2 moles
17g
= molar volume = 22.4 L = 0.2 moles x 22.4L
1 mole 1 mole
= Volume occupied by 3.4g of Ammonia at STP = 4.5 Litres.
Now here are some very helpful, useful links that can help you with this topic:
1) http://www.docbrown.info/page04/4_73calcs/MVGsaTEST.htm
2) http://www.fordhamprep.org/gcurran/sho/sho/lessons/lesson94.htm
3) http://www.fordhamprep.org/gcurran/sho/sho/lessons/lesson95.htm
4) http://jc-schools.net/dynamic/science/worksheets/MolarVolumeSTPPractice.pdf
5) http://www.savitapall.com/gases/assignments/Molar%20Volume.pdf
Now here are some images that will help you visually:

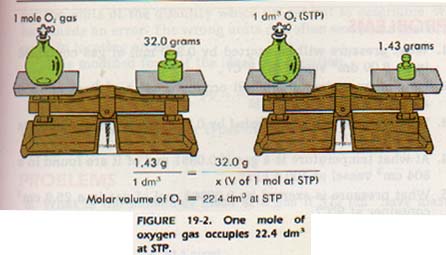

During class, we got a worksheet called "Chemistry: Molar Volume Worksheet" that should have been completed in class. This sheet is very good for review and to study from as those links above are also too.
For homework we have a sheet called "Chapter 4 Review WS" that needs to completed for next class (which is tomorrow). This is review for our test next week on Chapter 4 on Tuesday! That is all for now, so have a great day!
No comments:
Post a Comment