Thursday, January 27, 2011
January 27th
Synthesis is the forming of a compound from two or more simple substances.
When Oxygen (O2) first met Sodium (Na), both elements knew it was love at first sight, so they got together.
1 O2 + 4 Na ----> 2 Na2O
The general form for synthesis is A + B ----> C
Decomposition is the breaking apart of a single compound into more basic substances.
A few weeks into their relationship, Oxygen and Sodium began experiencing problems. She didn't think his jokes were funny. He never knew there were so many ways to ruin potato salad. They broke apart.
2Na2O ----> 1 O2 + 4 Na
The general form for decomposition is A ----> B + C
Single Replacement is when a compound swaps one element or ion for another.
Oxygen began dating another element, Iron (III), to replace the hole Sodium left in her heart. However, after a while she began to see that nobody could ever replace Sodium. One day she decided to call Sodium up, and soon the two of them were in love again.
Oxygen dumped Iron (III) and replaced him with Sodium again.
1 Fe2O3 + 6 Na ----> 3 Na2O + 2 Fe
The general form for single replacement is
A + BC ----> AC + B (when A is a metal)
A + BC ----> BA + C (when A is a non-metal)
AND THEY ALL LIVED HAPPILY EVER AFTER
(except for Iron (III). Poor guy.)
Note: Only more reactive metals can replace less reactive metals in single replacement reactions...and only more reactive non-metals can replace less reactive non-metals. So if Oxygen decided to date, say...Lithium, Sodium would not be able to win her back. Use your Activity Series sheet to determine when reactions occur.
Tuesday, January 25, 2011
January 25th
Balancing Equations
Why do we balance equations?
We balance equations because of the Law of the Conservation of Matter which states that
MATTER CANNOT BE CREATED OR DESTROYED IN A CHEMICAL REACTION
This means that, for example, if you have 5 atoms of iron to begin with as a reactant, you will have 5 atoms of iron in the end product. That's why we need to balance our equations!
Balance THIS!
__ Cl2 + __ LiI à __ LiCl + __ I2
TIP: Balance the single elements last, as they are the easiest ones to work with (you want to balance the hard stuff first!) However, it might be easier in this case to look at the elements that exist naturally with a 2 subscript (the "Diatomic 7" - Nitrogen, Oxygen, Fluorine, Chlorine, Bromine, and Iodine) and work with them.
__ Cl2 + 2 LiI à 2 LiCl + __ I2
Now balance the single elements.
1 Cl2 + 2 LiI à 2 LiCl + 1 I2
Remember, even if there's only one of an element, you still put the "1" in front when you're balancing.
Balance THIS!
__ FeBr3 + __ (NH4)2 S à __ Fe2S3 + __ NH4Br
TIP: Balance bigger "chunks" whenever you are able. The NH4 above, for example, can be seen as one whole instead of as so-and-so many N's and H's (makes for less of a hassle).
__ FeBr3 + 3 (NH4)2 S à __ Fe2S3 + 6 NH4Br (we use 3 and 6 because we have already been given 3 Br's on the reactant side)
2 FeBr3 + 3 (NH4)2 S à __ Fe2S3 + 6 NH4Br
2 FeBr3 + 3 (NH4)2 S à 1 Fe2S3 + 6 NH4Br
THE BALANCING GAME
Maybe one day your balancing skills will be as good as his! Hehe...
Wednesday, January 19, 2011
January 19th
Sadly, we did not get a proper lesson in chemistry today because we had an assembly. It was on internet safety, and held everyone's interest for a long, long time.
Oh my goodness this is exciting.
After we got back, everyone picked up two worksheets:
- Translating Word Equations Sheet #1-10
- Review of Naming Ionic and Covalent Compounds Sheet
Sunday, January 16, 2011
January 13
Hahahaha,,, Herroooooo everyone XDDD
How's going for the weekend?? Well,,, hope all of you have fun and have studied for Chemistry!!!!!!
Coz,, do you remember???? WE HAVE A TEST ON THE !17TH!!!!!!!!! OMG!!!
Anyways,,,,, Just let me type in all the needed formulas first~~~ XDDDD
STP: 22.4 L of gas/ 1 mole of gas or,,,,,,,,,,,,, 1 mole of gas/ 22.4 L of gas
Dilution: M1L1=M2L2
Molarity: moles of solute(mol) / Volume of solution (L) (M=mol/L)
mol= M x L L=mol/M
HAHAHAHHAHAHA,,,,,, here,,, some websites for review =]]]] XDD
http://www.chem.tamu.edu/class/majors/tutorialnotefiles/empirical.htm
http://faculty.mdc.edu/avelazq1/MOLECULAR%20AND%20EMPIRICAL%20FORMULAS%20version%202.htm
http://dl.clackamas.cc.or.us/ch105-04/calculat.htm
http://science.widener.edu/svb/tutorial/molarity2csn7.html
Good Luck~ =]
How's going for the weekend?? Well,,, hope all of you have fun and have studied for Chemistry!!!!!!
Coz,, do you remember???? WE HAVE A TEST ON THE !17TH!!!!!!!!! OMG!!!
Anyways,,,,, Just let me type in all the needed formulas first~~~ XDDDD
STP: 22.4 L of gas/ 1 mole of gas or,,,,,,,,,,,,, 1 mole of gas/ 22.4 L of gas
Dilution: M1L1=M2L2
Molarity: moles of solute(mol) / Volume of solution (L) (M=mol/L)
mol= M x L L=mol/M
HAHAHAHHAHAHA,,,,,, here,,, some websites for review =]]]] XDD
http://www.chem.tamu.edu/class/majors/tutorialnotefiles/empirical.htm
http://faculty.mdc.edu/avelazq1/MOLECULAR%20AND%20EMPIRICAL%20FORMULAS%20version%202.htm
http://dl.clackamas.cc.or.us/ch105-04/calculat.htm
http://science.widener.edu/svb/tutorial/molarity2csn7.html
Good Luck~ =]
Wednesday, January 12, 2011
January 11, 2011
Why Hello, Hello, Hello!! I hope everybody is in good spirit and is enjoying the weather?!?! Maybe, maybe not? I know that I am definetely NOT!! Anyway enough about the cold weather and lets talk some Chemistry! Woooo!!
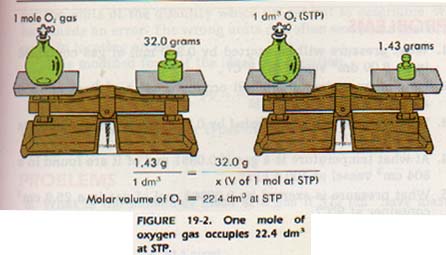
Today in class we took notes of the....... Molar Volume of a Gas at STP
- Description of a STP: The definition is that STP is a standard condition that compares volume of gases. STP stands for Standard Temperature & Pressure. A STP is one atmosphere of pressure and a temperature of 0 degrees celsius and 273.1 K. At STP one mole of gas = 22.4 L.
- The two CONVERSION FACTORS:
1. 22.4L of gas 2. 1 mole of gas
1 mole of gas 22.4L of gas
Here is an example for you to practice:
1) calculate the volume occupied by 3.4g of ammonia at STP
= molar mass of ammonia (NH3) = 17 g/mole
= 3.4g x 1 mole = 0.2 moles
17g
= molar volume = 22.4 L = 0.2 moles x 22.4L
1 mole 1 mole
= Volume occupied by 3.4g of Ammonia at STP = 4.5 Litres.
Now here are some very helpful, useful links that can help you with this topic:
1) http://www.docbrown.info/page04/4_73calcs/MVGsaTEST.htm
2) http://www.fordhamprep.org/gcurran/sho/sho/lessons/lesson94.htm
3) http://www.fordhamprep.org/gcurran/sho/sho/lessons/lesson95.htm
4) http://jc-schools.net/dynamic/science/worksheets/MolarVolumeSTPPractice.pdf
5) http://www.savitapall.com/gases/assignments/Molar%20Volume.pdf
Now here are some images that will help you visually:


During class, we got a worksheet called "Chemistry: Molar Volume Worksheet" that should have been completed in class. This sheet is very good for review and to study from as those links above are also too.
For homework we have a sheet called "Chapter 4 Review WS" that needs to completed for next class (which is tomorrow). This is review for our test next week on Chapter 4 on Tuesday! That is all for now, so have a great day!
Today in class we took notes of the....... Molar Volume of a Gas at STP
- Description of a STP: The definition is that STP is a standard condition that compares volume of gases. STP stands for Standard Temperature & Pressure. A STP is one atmosphere of pressure and a temperature of 0 degrees celsius and 273.1 K. At STP one mole of gas = 22.4 L.
- The two CONVERSION FACTORS:
1. 22.4L of gas 2. 1 mole of gas
1 mole of gas 22.4L of gas
Here is an example for you to practice:
1) calculate the volume occupied by 3.4g of ammonia at STP
= molar mass of ammonia (NH3) = 17 g/mole
= 3.4g x 1 mole = 0.2 moles
17g
= molar volume = 22.4 L = 0.2 moles x 22.4L
1 mole 1 mole
= Volume occupied by 3.4g of Ammonia at STP = 4.5 Litres.
Now here are some very helpful, useful links that can help you with this topic:
1) http://www.docbrown.info/page04/4_73calcs/MVGsaTEST.htm
2) http://www.fordhamprep.org/gcurran/sho/sho/lessons/lesson94.htm
3) http://www.fordhamprep.org/gcurran/sho/sho/lessons/lesson95.htm
4) http://jc-schools.net/dynamic/science/worksheets/MolarVolumeSTPPractice.pdf
5) http://www.savitapall.com/gases/assignments/Molar%20Volume.pdf
Now here are some images that will help you visually:



During class, we got a worksheet called "Chemistry: Molar Volume Worksheet" that should have been completed in class. This sheet is very good for review and to study from as those links above are also too.
For homework we have a sheet called "Chapter 4 Review WS" that needs to completed for next class (which is tomorrow). This is review for our test next week on Chapter 4 on Tuesday! That is all for now, so have a great day!
Friday, January 7, 2011
Diluting Solutions
"Hi, fellow students... unfortunately, the blogger today had an accident with her experiment involving diluting solutions! In the mean time, I will be teaching you guys all about this lesson because obviously I know more than her to start with. She made many dumb mistakes in preparing for workable solutions indeed!"
Diluting solutions is actually a simple math involving the formula we have learned....Molarity M = mole/ litre
The KEY CONCEPT of diluting solutions is:
[MOLES of Solutes is constant before, after and forever...]
Moles of solute b4 = moles of solute after
How to get moles?
M x L !
thus, M1L1 = M2L2
(careful, 2 meaning the second solute, not times 2)
I just don't understand how she could not do this simple calculation to get the solution right...keke...I am sure all of you can do the following examples as fast as I can do.
Eg. I have 2 Litre of 10 M of Hcl, and I need 0.5 Litre of 1 M Hcl.
Consider if I need all of 10 M of Hcl? No..
I need to solve for how much Volume to start with ...L1
M1L1 = M2L2
10 M x L1 = 1M x 0.5L
L1 = 0.05 L
So if I need 0.05 L of the 10 M Hcl solution to make 0.5 L of 1M Hcl... I would take 0.05 L of concentrated Hcl and add how much water?
0.5 - 0.05 = 0.45 L
These are useful practices to get familiar with the calculation:
note: here V = the volume in Litre!
- M2=(M1V1) ÷ V2
- M1 = 0.25M
- V1 = 100mL = 100 ÷ 1000 = 0.100L (volume must be in litres)
- V2 = 1.5L
- [NaCl(aq)]new = M2 = (0.25 x 0.100) ÷ 1.5 = 0.017M
(or 0.0.017 mol/L or 0.0.17mol L-1)
- V2=(M1V1) ÷ M2
- M1 = 0.02M
- V1 = 500mL = 500 ÷ 1000 = 500 x 10-3L = 0.500L (since there are 1000mL in 1L)
- M2 = 0.001M
- V(CuSO4)new = V2 = (0.02 x 0.500) ÷ 0.001 = 10.00L
Wednesday, January 5, 2011
Subscribe to:
Comments (Atom)




















