Hahaha,,,, today,, we did a experiment,,,
which is lab 4C FORMULA OF A HYDRATE.
The procedure was:
First,,, Wear your safety GOGGLES!!
Then set up the pipestem triangle, iron ring, stsand and bunsen burner....
Heat the crucible with busen burner for around 3 min,, to make sure it is dry,,,,,,
-Let crucible cool down and measure the mass of it.
-put the given hydrate inside the crucible and record the mass.
_heat the crucible(with hydrate) until the buttom of crucible is dull red,,, for about five min
-after that,, let it cool down for approximately five min,,,, and record down the mass of it
-to make sure all water is driven off,,,, reheat it for another five min
-cool it down for another five min again and record the mass
-lastly,,,, drops a few water to the contents of the crucible and see what happen!! XDD
Links:
Another experiment for formula of a hydrate:
http://chem.lapeer.org/Chem1Docs/HydrateLab1.html
A video clip for the expriment:
http://www.youtube.com/watch?v=OuF4hjTFdsg
HAHAHA,, That's all.....
Have A Nice Day! ^^ XDD
Tuesday, December 7, 2010
Friday, December 3, 2010
DECEMBER 3rd
Do you remember the CH compounds or the compounds containing Carbon?
They are referred to as the ORGANIC COMPOUND--
The Question is...
How do you calculate the Empirical formula of Organic Compounds?

For example, given:
They are referred to as the ORGANIC COMPOUND--
The Question is...
How do you calculate the Empirical formula of Organic Compounds?
For example, given:
What's the empirical formula of a compound when a 5.00 gram sample is burned producing 15.0 grams of CO2 and 8.18 g of H2O?
Let the empirical formula of the compound be CxHy.
CxHy + zO2 -> xCO2 + y/2 H2O
1. Find the moles of C and H in the compound.
CO2: 15.0 g x 1mol/44.0 g = 0.341 mol
H2O: 8.18 x 1mol/18.02g = 0.454 mol
2. 0.341 moles of C 0.341/0.341 = 1 x 3 =3
0.454 x 2 = 0.908moles of H 0.908/0.341 = 2.66 x 3 = 8
3. Ratio of C:H = 3:8
therefore, the empirical formula is C3H8.
4. Now, check the masses:
0.341 mol of C x 12g/1mol = 4.092 g of C
0.908 mol of C x 1.0g.1mol = 0.908 g of H
Total mass = 5.00 g
Therefore, there is no Oxygen atom in the compound.
*Here is a challenging question for an extra thinking~
Adipic acid is an organic compound composed of 49.31% C, 43.79% O, and the rest hydrogen. If the molar mass of adipic acid is 146.1g/mol, what are the empirical and molecular formulas for adipic acid?
if there were 100 g of the sample, it would contain
49.31 g C
43.79 g O
6.90 g H
convert everything to moles
49.31 g C------>4.11 mol C
43.79 g O------>2.74 mol O
6.90 g H -------->6.83 mol H
now, we have the molar ratio
C:O:H = 4.11 mol C : 2.74 mol O: 6.83 mol H
reduce the ratio to form in which the smallest term is 1
do this by dividing each term by the smallest value (2.74)
4.11 mol C : 2.74 mol O: 6.83 mol H = 1.5 mol C : 1 mol O :2.5 mol H
to eliminate fractions, multiply by the LCD (2 in this case)
1.5 mol C : 1 mol O :2.5 mol H = 3 mol C : 2 mol O :5 mol H
thus, the empirical formula is C3H5O2
======================================…
to find the molecular formula, we have to know how many times the empirical weight is the molecular weight
we do this by dividing the molecular weight by th empirical weight
Empirical Weight (EW)
=(12 g/mol C)(3 mol C) + (1.01 g/mol H)(5 mol H) + (16 g/mol O)(2 mol O)
= 73.05 g
Molecular Weight (MW) = 146.1 g
MW / EW = 146.1 g / 73.05 g = 2
multiply this by the subscripts of the elements in the empirical formula
===============
thus, the molecular formula is C6H10O4
thus, the empirical formula is C3H5O2
Did you get it??
49.31 g C
43.79 g O
6.90 g H
convert everything to moles
49.31 g C------>4.11 mol C
43.79 g O------>2.74 mol O
6.90 g H -------->6.83 mol H
now, we have the molar ratio
C:O:H = 4.11 mol C : 2.74 mol O: 6.83 mol H
reduce the ratio to form in which the smallest term is 1
do this by dividing each term by the smallest value (2.74)
4.11 mol C : 2.74 mol O: 6.83 mol H = 1.5 mol C : 1 mol O :2.5 mol H
to eliminate fractions, multiply by the LCD (2 in this case)
1.5 mol C : 1 mol O :2.5 mol H = 3 mol C : 2 mol O :5 mol H
thus, the empirical formula is C3H5O2
======================================…
to find the molecular formula, we have to know how many times the empirical weight is the molecular weight
we do this by dividing the molecular weight by th empirical weight
Empirical Weight (EW)
=(12 g/mol C)(3 mol C) + (1.01 g/mol H)(5 mol H) + (16 g/mol O)(2 mol O)
= 73.05 g
Molecular Weight (MW) = 146.1 g
MW / EW = 146.1 g / 73.05 g = 2
multiply this by the subscripts of the elements in the empirical formula
===============
thus, the molecular formula is C6H10O4
thus, the empirical formula is C3H5O2
Did you get it??
Here is a summary of the steps
1. Find # of moles of the compounds containing C and H
2. Find the # of moles of atom of C, H
3. Determine the ratio of C:H based on the # of moles (divide by the smallest number)
4. IF there are decimals, use either rounding or multiply to get a whole number
5. FINALLY, PLEASE check the masses of each atom of C and H to see if it equals the mass (if given) in the question. If it does not, then there must be a O atom in the compound. Find the mass of O atom and convert to moles in order to determine the C:H:O ratio!
Wednesday, December 1, 2010
December 1, 2010
Wow, I almost can't believe it, it is already the start of December!
Today is officially December 1st so that means it is 24 days until Christmas & 17 more days until Winter Vacation! We are almost there, so keep going everybody!!
Well enough about Christmas (which is my favourite holiday) and lets talk Science.
Today we learned a new topic..... are you ready for this?..........
Empirical + Molecular Formaula!!!!!!
Now this topic is very detailed so stay with me here;
Lets first start with Empirical Formula; (EF)
- EF is the lowest term ratio of atoms in a formula. Otherwise known as reducing.
ex. C6H12 = molecular formula
C3H6 = CH2 = empirical formala
ex. consider that we have 10.87g of Fe and 4.66g of O. What is the EF?
1st Step. Convert grams to moles
Fe 10.87g x 1 mol / 55.8g = 0.195 mols of Fe
O 4.66g x 1 mol / 16.0g = 0.291 mols of O
2nd Step. Divide both by the smaller molar amount
Fe 0.195 / 0.195 = 1
O 0.291 / 0.195 = 1.5
3rd Step. Scale ratios to whole numbers
Fe 1 x 2 = 2
O 1.5 x 2 = 3 Therefore the answer is: Fe2O3
ex. A compund contains 31.9 % K, 28.9 % Cl, 39.2 % O. What is the EF?
* Assume you have 100g
1st Step. Convert grams to moles
K 31.9g x 1 mol / 39.1g = 0.816 mol
Cl 28.9g x 1 mol / 35.5g = 0.814 mol
O 39.2g x 1 mol / 16g = 2.45 mol
2nd Step. Divide each by the smaller molar amount
K 0.816 / 0.814 = 1
Cl 0.814 / 0.814 = 1
O 2.45 / 0.814 = 3
3rd Step. Scale ratios to whole numbers
Dont need to do because all ratios are whole numbers already (1, 1, 3)
Therefore the answer is: KClO3
Now I will explain Molecular Formula; (MF)
- MF is the multiple of empirical forms. It shows the number of atoms that combine to form a molecule.
- Formula: n = mm of the compund / mm of the empirical formula. (mm = molar mass)
ex. A molecule has an empirical formula of C2H5 and a molar mass of 58 g/mol.
What is the MF?
MM of C2H5 = 29.0 g/mol
n = 58 g/mol
----------- = 2
29.0 g/mol
MF = 2(C2H5) = C4H10
ex. The empirical formula of a gas is CH2. What is the MF if MM is 42 g/mol?
MM of CH2 = 14 g/mol
n = 42 g/mol
----------- = 3
14 g/mol
MF = 3(CH2) = C3H6
ex. A compound contains 7.44g of C, 1.24g of H, 9.92g of O. The MM is 180 g/mol. What is the MF?
1st Step. Find the empirical formula since it is not shown in the question.
C 7.44 x 1 mol / 12g = 0.62 mols
H 1.24 x 1 mol / 1g = 1.24 mols
O 9.92 x 1 mol / 16g = 0.62 mols
2nd Step. Then divide each by the smallest molar amount which is 0.62
C = 1 H = 2 O = 1 therefore EF = CH2O
3rd Step. Now you can find the MF.
MM of CH2O = 30 g/mol
n = 180 g/mol
----------- = 6
30 g/mol
MF = 6(CH2O) = C6H12O6
That concludes of all what we did today, now I see that in the last blog Percent Composition was not explained so I will explain that right now. Percent Compostion is what we did last class.
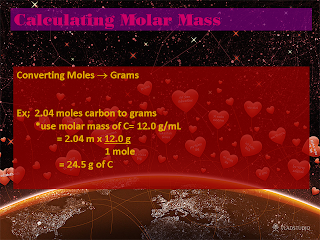
Percent Composition: (PC)
- PC is percentage by mass of a "species" in a chemical formula.
ex. What is the PC of CO2?
*Assume you have one mole
Total MM = 44.0 g/mole
% of C = 12.0 divided by 44.0 x 100% = 27.3 %
% of O = 32 divided by 44 x 100% = 72.7 %
Notice that they both add up to a hundred percent, in these equations the two or three answers should always add up to a hundred percent or at least very close to.
ex. Compound contains 5.1g of Cl, 22.0g of C, and has a total mass of 44.1g and contains some amount of O. Calculate the PC.
Mass of Oxygen = 44.1 - 5.1 - 22.0 = 17.0 g
% of Cl = 5.1 divided by 44.1 x 100% = 11.6 = 12%
% of C = 22.0 divided by 44.1 x 100% = 49.9%
% of O = 17.0 divided by 44.1 x 100% = 38.5%
All add up to 100.4 percent. This is a way to check your work too by the way.
ex. Calculate the PC of the underlined components of (NH4)2SO3
Total MM = 116.1
MM of all the NH4 = 36
% of NH4 = 36 divided by 116.1 x 100% = 31.0%
Whew, alot of numbers as you can see :). Now its time to treat you with some video's & practice questions for your own practice!
- Worksheets:
Empirical + Molecular Formula
1) http://eaglepoint.lexingtonchristian.org/hs/science/dhumphreys/Shared%20Documents/Honors%20Chemistry/Empirical%20Formula%20Worksheet%205.pdf
2http://www.ccboe.net/Teachers/nutial_laura/files/CBDD907F339B420CB25588150BD481A9.doc
3) http://www.ezclasssites.com/data/captainkirk/EmpiricalFormulaWS.pdf
4) http://www.fordhamprep.org/gcurran/sho/sho/lessons/lesson59.htm
5) http://lhs2.lps.org/staff/sputnam/practice/UnitV_EmpForm.htm
6) http://westwood.sjsd.net/~dshoesmith/FOV1-0003789A/FOV1-000378A1/Percent%20Composition%20and%20Molecular%20Formula%20Worksheet.doc?FCItemID=S01C8C50E
7) http://www.cbv.ns.ca/rv/campbell/Resources/M%20F%20EF.pdf
Percentage Composition
1) http://misterguch.brinkster.net/PRA023.pdf
2) http://www.fordhamprep.org/gcurran/sho/sho/worksheets/worksht58a.htm
3) http://www.fordhamprep.org/gcurran/sho/sho/lessons/lesson58.htm
4) http://misterguch.brinkster.net/001_024.doc
5http://cmsweb1.loudoun.k12.va.us/52820831134912597/lib/52820831134912597/Moles/Homework/masspercomp.pdf
6http://www.ccboe.net/Teachers/epperson_steve/files/36F0A3C2EC0648DBB63EF8981578B7A0.doc
7http://asd1.schoolwires.com/1741206142323657/lib/1741206142323657/Percentage_Composition_Worksheet.pdf
- Videos
1) http://www.youtube.com/watch?v=xbEeyT8nK84
2) http://www.youtube.com/watch?v=_H009sTvYE0
3) http://www.youtube.com/watch?v=gfBcM3uvWfs
Ooops almost forgot...... HW:
1) Empirical and Molecular Formula
2) Reminder: Quiz next week regarding this material and the material will be covered on Frida (next class).
Thank You and Have A Great Weekend!!!!!
Today is officially December 1st so that means it is 24 days until Christmas & 17 more days until Winter Vacation! We are almost there, so keep going everybody!!
Well enough about Christmas (which is my favourite holiday) and lets talk Science.
Today we learned a new topic..... are you ready for this?..........
Empirical + Molecular Formaula!!!!!!
Now this topic is very detailed so stay with me here;
Lets first start with Empirical Formula; (EF)
- EF is the lowest term ratio of atoms in a formula. Otherwise known as reducing.
ex. C6H12 = molecular formula
C3H6 = CH2 = empirical formala
ex. consider that we have 10.87g of Fe and 4.66g of O. What is the EF?
1st Step. Convert grams to moles
Fe 10.87g x 1 mol / 55.8g = 0.195 mols of Fe
O 4.66g x 1 mol / 16.0g = 0.291 mols of O
2nd Step. Divide both by the smaller molar amount
Fe 0.195 / 0.195 = 1
O 0.291 / 0.195 = 1.5
3rd Step. Scale ratios to whole numbers
Fe 1 x 2 = 2
O 1.5 x 2 = 3 Therefore the answer is: Fe2O3
ex. A compund contains 31.9 % K, 28.9 % Cl, 39.2 % O. What is the EF?
* Assume you have 100g
1st Step. Convert grams to moles
K 31.9g x 1 mol / 39.1g = 0.816 mol
Cl 28.9g x 1 mol / 35.5g = 0.814 mol
O 39.2g x 1 mol / 16g = 2.45 mol
2nd Step. Divide each by the smaller molar amount
K 0.816 / 0.814 = 1
Cl 0.814 / 0.814 = 1
O 2.45 / 0.814 = 3
3rd Step. Scale ratios to whole numbers
Dont need to do because all ratios are whole numbers already (1, 1, 3)
Therefore the answer is: KClO3
Now I will explain Molecular Formula; (MF)
- MF is the multiple of empirical forms. It shows the number of atoms that combine to form a molecule.
- Formula: n = mm of the compund / mm of the empirical formula. (mm = molar mass)
ex. A molecule has an empirical formula of C2H5 and a molar mass of 58 g/mol.
What is the MF?
MM of C2H5 = 29.0 g/mol
n = 58 g/mol
----------- = 2
29.0 g/mol
MF = 2(C2H5) = C4H10
ex. The empirical formula of a gas is CH2. What is the MF if MM is 42 g/mol?
MM of CH2 = 14 g/mol
n = 42 g/mol
----------- = 3
14 g/mol
MF = 3(CH2) = C3H6
ex. A compound contains 7.44g of C, 1.24g of H, 9.92g of O. The MM is 180 g/mol. What is the MF?
1st Step. Find the empirical formula since it is not shown in the question.
C 7.44 x 1 mol / 12g = 0.62 mols
H 1.24 x 1 mol / 1g = 1.24 mols
O 9.92 x 1 mol / 16g = 0.62 mols
2nd Step. Then divide each by the smallest molar amount which is 0.62
C = 1 H = 2 O = 1 therefore EF = CH2O
3rd Step. Now you can find the MF.
MM of CH2O = 30 g/mol
n = 180 g/mol
----------- = 6
30 g/mol
MF = 6(CH2O) = C6H12O6
That concludes of all what we did today, now I see that in the last blog Percent Composition was not explained so I will explain that right now. Percent Compostion is what we did last class.
Percent Composition: (PC)
- PC is percentage by mass of a "species" in a chemical formula.
ex. What is the PC of CO2?
*Assume you have one mole
Total MM = 44.0 g/mole
% of C = 12.0 divided by 44.0 x 100% = 27.3 %
% of O = 32 divided by 44 x 100% = 72.7 %
Notice that they both add up to a hundred percent, in these equations the two or three answers should always add up to a hundred percent or at least very close to.
ex. Compound contains 5.1g of Cl, 22.0g of C, and has a total mass of 44.1g and contains some amount of O. Calculate the PC.
Mass of Oxygen = 44.1 - 5.1 - 22.0 = 17.0 g
% of Cl = 5.1 divided by 44.1 x 100% = 11.6 = 12%
% of C = 22.0 divided by 44.1 x 100% = 49.9%
% of O = 17.0 divided by 44.1 x 100% = 38.5%
All add up to 100.4 percent. This is a way to check your work too by the way.
ex. Calculate the PC of the underlined components of (NH4)2SO3
Total MM = 116.1
MM of all the NH4 = 36
% of NH4 = 36 divided by 116.1 x 100% = 31.0%
Whew, alot of numbers as you can see :). Now its time to treat you with some video's & practice questions for your own practice!
- Worksheets:
Empirical + Molecular Formula
1) http://eaglepoint.lexingtonchristian.org/hs/science/dhumphreys/Shared%20Documents/Honors%20Chemistry/Empirical%20Formula%20Worksheet%205.pdf
2http://www.ccboe.net/Teachers/nutial_laura/files/CBDD907F339B420CB25588150BD481A9.doc
3) http://www.ezclasssites.com/data/captainkirk/EmpiricalFormulaWS.pdf
4) http://www.fordhamprep.org/gcurran/sho/sho/lessons/lesson59.htm
5) http://lhs2.lps.org/staff/sputnam/practice/UnitV_EmpForm.htm
6) http://westwood.sjsd.net/~dshoesmith/FOV1-0003789A/FOV1-000378A1/Percent%20Composition%20and%20Molecular%20Formula%20Worksheet.doc?FCItemID=S01C8C50E
7) http://www.cbv.ns.ca/rv/campbell/Resources/M%20F%20EF.pdf
Percentage Composition
1) http://misterguch.brinkster.net/PRA023.pdf
2) http://www.fordhamprep.org/gcurran/sho/sho/worksheets/worksht58a.htm
3) http://www.fordhamprep.org/gcurran/sho/sho/lessons/lesson58.htm
4) http://misterguch.brinkster.net/001_024.doc
5http://cmsweb1.loudoun.k12.va.us/52820831134912597/lib/52820831134912597/Moles/Homework/masspercomp.pdf
6http://www.ccboe.net/Teachers/epperson_steve/files/36F0A3C2EC0648DBB63EF8981578B7A0.doc
7http://asd1.schoolwires.com/1741206142323657/lib/1741206142323657/Percentage_Composition_Worksheet.pdf
- Videos
1) http://www.youtube.com/watch?v=xbEeyT8nK84
2) http://www.youtube.com/watch?v=_H009sTvYE0
3) http://www.youtube.com/watch?v=gfBcM3uvWfs
Ooops almost forgot...... HW:
1) Empirical and Molecular Formula
2) Reminder: Quiz next week regarding this material and the material will be covered on Frida (next class).
Thank You and Have A Great Weekend!!!!!
Friday, November 26, 2010
25th November, 2010
Well Hi there~~
Today,,,,, we just finished our quiz on mole conversions,,,,,, sooooo,, actually,,
WE DIDN"T LEARN ANY NEW THING TODAY!!! =[
Anyways,,,,, I think doing a revision about mole conversions here would be better before we start on something new~~
So here,,,,
Converting gram to mole is : x1mole/MMg
Moles to particles: x 6.022x10^23/1 mole
Particles to #atoms in particle: x #of atoms/1 molecule
P.S> don't forget to that we have homework.
the two worksheets: The wonderful world of mole
Harder mole conversions!
Have a fun weekend. =]
Well Hi there~~
Today,,,,, we just finished our quiz on mole conversions,,,,,, sooooo,, actually,,
WE DIDN"T LEARN ANY NEW THING TODAY!!! =[
Anyways,,,,, I think doing a revision about mole conversions here would be better before we start on something new~~
So here,,,,
Converting gram to mole is : x1mole/MMg
Moles to particles: x 6.022x10^23/1 mole
Particles to #atoms in particle: x #of atoms/1 molecule
P.S> don't forget to that we have homework.
the two worksheets: The wonderful world of mole
Harder mole conversions!
Have a fun weekend. =]
Tuesday, November 23, 2010
THE HOLY MOLY CONVERSIONS~
HOLY MOLY! now, we are talking about Harder Mole Conversions...
Well, they aren't necessarily much harder. If you master the basic stuff, this should be easy!
One thing you HAVE to keep in memory is THIS: MOLE MAP!-> you will never get lost with it!
Now, recall that MOLE is defined as the amount of substance that contains 6.022 X 10^23 atoms or molecules or subatomic particles. This numeric value 6.022 X 10^23 is also called as Avogadro's number.
So, a mole of any substance (atom or molecule) would be equal to the atomic mass/ molecular mass of that substance in gram.
Steps to be followed:
1) Find the molar mass of element
2) Conversion of mass ( 80g ) into moles.
3) Getting the multiplication of obtained moles by avogadro's number to get the number of atoms.
Since we Know that
Atomic mass of Ca= 40 g/mole
so, the number of moles in 80g of Ca = given mass / atomic mass
= 80g / 40 g/mol = 2 moles
Here we got the 2 moles of Ca from 80g of Ca.
Now, 1 mole of Ca = 6.022 x 10^23 atoms of Ca ( By definition )
Therefore, 2 moles of Ca = 2 X 6.022 X 10^23 atoms
= 1.204 X 10^24 atoms of Ca
Thus, we got that
80g of calcium contains 1.204 X 10^24 atoms of the substance Ca.
Steps to be followed:
1) Find the molecular mass of substance ( here, 40g / mole)
2) Find the moles of this mass
3) convert this moles into number of molecules of substance
4) get the individual number of constituent elementary atoms
Here we know the molar mass of NaOH= 40 g/mole
So, the no. of moles in 80g of NaOH = 80g/ 40 g/mole
= 2 moles
Now, since 1 mole of NaOH = 6.022 X 10^23 molecules of NaOH
So, 2 moles of NaOH = 2* 6.022 x 10^23 molecules of NaOH = 1.204 X 10^24 molecules of NaOH
Now, calculating the individual constituents elementary atoms
Constituents 1 molecule of NaOH 1.204 X 10^24 molecules of NaOH
Na 1 1* 1.204 x 10^24 = 1.204 X 10^24 atoms of Na
O 1 1* 1.204 x 10^24 = 1.204 X 10^24 atoms of O
H 1 1* 1.204 x 10^24 = 1.204 X 10^24 atoms of H
THe MOLE mAP!~
x 1mol/MMg x Avagadro's# p./1mole x # atoms/1 molecules
Grams ------------> Moles -------------------> Ptcl-------># of atoms
x 1molecules/# of atoms x 1mol/Avagadro's# x 1 mol/MMG
# of atoms -----> Ptcl-------> Moles ------> Grams
Basically follow the map step by step, never try to be smart and skip one step!!

Well, they aren't necessarily much harder. If you master the basic stuff, this should be easy!
One thing you HAVE to keep in memory is THIS: MOLE MAP!-> you will never get lost with it!
Now, recall that MOLE is defined as the amount of substance that contains 6.022 X 10^23 atoms or molecules or subatomic particles. This numeric value 6.022 X 10^23 is also called as Avogadro's number.
So, a mole of any substance (atom or molecule) would be equal to the atomic mass/ molecular mass of that substance in gram.
Calculation1: for Element: Grams to Moles to Atoms
Lets calculate the number of atoms in 80g of calcium.Steps to be followed:
1) Find the molar mass of element
2) Conversion of mass ( 80g ) into moles.
3) Getting the multiplication of obtained moles by avogadro's number to get the number of atoms.
Since we Know that
Atomic mass of Ca= 40 g/mole
so, the number of moles in 80g of Ca = given mass / atomic mass
= 80g / 40 g/mol = 2 moles
Here we got the 2 moles of Ca from 80g of Ca.
Now, 1 mole of Ca = 6.022 x 10^23 atoms of Ca ( By definition )
Therefore, 2 moles of Ca = 2 X 6.022 X 10^23 atoms
= 1.204 X 10^24 atoms of Ca
Thus, we got that
80g of calcium contains 1.204 X 10^24 atoms of the substance Ca.
Calculation 2: for Compound: Grams to Moles to Atoms
Lets calculate the no. of atoms in a compound, say 80g NaOHSteps to be followed:
1) Find the molecular mass of substance ( here, 40g / mole)
2) Find the moles of this mass
3) convert this moles into number of molecules of substance
4) get the individual number of constituent elementary atoms
Here we know the molar mass of NaOH= 40 g/mole
So, the no. of moles in 80g of NaOH = 80g/ 40 g/mole
= 2 moles
Now, since 1 mole of NaOH = 6.022 X 10^23 molecules of NaOH
So, 2 moles of NaOH = 2* 6.022 x 10^23 molecules of NaOH = 1.204 X 10^24 molecules of NaOH
Now, calculating the individual constituents elementary atoms
Constituents 1 molecule of NaOH 1.204 X 10^24 molecules of NaOH
Na 1 1* 1.204 x 10^24 = 1.204 X 10^24 atoms of Na
O 1 1* 1.204 x 10^24 = 1.204 X 10^24 atoms of O
H 1 1* 1.204 x 10^24 = 1.204 X 10^24 atoms of H
THe MOLE mAP!~
x 1mol/MMg x Avagadro's# p./1mole x # atoms/1 molecules
Grams ------------> Moles -------------------> Ptcl-------># of atoms
x 1molecules/# of atoms x 1mol/Avagadro's# x 1 mol/MMG
# of atoms -----> Ptcl-------> Moles ------> Grams
Basically follow the map step by step, never try to be smart and skip one step!!

Monday, November 22, 2010
November 19, 2010
Mole Conversions !!!
Ahhhhh, good old mole conversions, you yet to surprise me again... ;)
Well, to mix up today's lesson I will be presenting you a powerpoint!!! Woo, Yea!!!
And this is no other normal powerpoint, noooo this is a powerpoint that will amaze your eyes and give you, yes you, important information that will be useful to what you need to know about Mole Conversions!
But first here are some videos that will also help you understand......
1) http://www.youtube.com/watch?v=_Od-lhWTG3Y&feature=fvst
2) http://www.youtube.com/watch?v=ehepBBtSbDc
3) http://www.youtube.com/watch?v=QcEyl4sG-Ac&feature=fvw
4) http://www.youtube.com/watch?v=NMdN1LtHuDA&feature=related
And here are some worksheets + questions.......
1) http://www.fordhamprep.org/gcurran/sho/sho/lessons/lesson92.htm
2) http://www.sciencegeek.net/Chemistry/taters/Unit4GramMoleVolume.htm
Powerpoint.......
(I could not save it into like a powerpoint presentation so instead down below are all of the slides in order)
Dont forget HW.......
1) Textbook pg. 104 # 4-9
2) Textbook pg. 109 # 4-16
3) Sheet - Mole Conversions Exercises; Excercise A-D
4) Sheet - Atomic Mass, Formula Mass & Molar Mass; Excercise B (Back Side)
Ahhhhh, good old mole conversions, you yet to surprise me again... ;)
Well, to mix up today's lesson I will be presenting you a powerpoint!!! Woo, Yea!!!
And this is no other normal powerpoint, noooo this is a powerpoint that will amaze your eyes and give you, yes you, important information that will be useful to what you need to know about Mole Conversions!
But first here are some videos that will also help you understand......
1) http://www.youtube.com/watch?v=_Od-lhWTG3Y&feature=fvst
2) http://www.youtube.com/watch?v=ehepBBtSbDc
3) http://www.youtube.com/watch?v=QcEyl4sG-Ac&feature=fvw
4) http://www.youtube.com/watch?v=NMdN1LtHuDA&feature=related
And here are some worksheets + questions.......
1) http://www.fordhamprep.org/gcurran/sho/sho/lessons/lesson92.htm
2) http://www.sciencegeek.net/Chemistry/taters/Unit4GramMoleVolume.htm
Powerpoint.......
(I could not save it into like a powerpoint presentation so instead down below are all of the slides in order)
Dont forget HW.......
1) Textbook pg. 104 # 4-9
2) Textbook pg. 109 # 4-16
3) Sheet - Mole Conversions Exercises; Excercise A-D
4) Sheet - Atomic Mass, Formula Mass & Molar Mass; Excercise B (Back Side)
Thursday, November 18, 2010
November 18th
A Mole Day Song! (Oh, won't you come and sing along?)
A mole is an animal that burrows in the ground,
Or a spot on your chin that you gotta shave around.
But there's another kind of mole of interest to me,
That's the kind of mole they use in chemistry.
Chorus: A mole is a unit, or have you heard,
Containing six times ten to the twenty-third,
That's a six with twenty-three zero's at the end,
Much too big a number to comprehend.
Say you had a mole of pennies to distribute 'round the world,
Give to each of the five billion grownups, boys, and girls,
There wouldn't be a single person down and out of luck,
Cause everybody in the world would get a trillion bucks.
Or say you had a mole of paper and stacked it toward the sky,
Paper's awful thin, but that pile would get so high.
It'd reach up into outer space, in fact I think you'd find,
It'd go up to the moon and back, eighty billion times.
Chorus: A mole is a unit, or have you heard,
Containing six times ten to the twenty-third,
That's a six with twenty-three zero's at the end,
Much too big a number to comprehend.
Suppose a mole of marshmallows fell upon the planet,
Over each square inch of land and sea, think that you could stand it?
That layer would be twelve miles high and of course block our sun,
We're talking close to five million trillion tons.
Well, maybe we could save ourselves if we all started eaten',
One marshmallow each second, not two 'cause that'd be cheatin',
With forty five billion people munching, how long do you think it'd take?
Forty million years, and that's without a bathroom break.
Chorus: A mole is a unit, or have you heard,
Containing six times ten to the twenty-third,
That's a six with twenty-three zero's at the end,
Much too big a number to comprehend.
But say you had a mole of atoms, would the pile be immense,
Should I say the answer now or leave you in suspense?
Well, atoms are so very small, very small, you understand,
You could hold a mole of atoms in the palm of your hand.
So shake a little sugar in the middle of your palm,
Now you don't want to spill it, so try and stay calm.
You hardly can imagine and barely realize,
There're more atoms in that sugar than stars up in the sky.
Chorus: A mole is a unit, or have you heard,
Containing six times ten to the twenty-third,
That's a six with twenty-three zero's at the end,
Much too big a number to comprehend.
Today's Class:
Atomic mass unit (amu): a way of measuring the mass of a single atom of an element. To find the amu of an element, look for its atomic mass on the periodic table.
For example, chlorine is 35.5 amu.
To find the amu of an ionic compound, add together the amu of each atom of that compound.
A mole is an animal that burrows in the ground,
Or a spot on your chin that you gotta shave around.
But there's another kind of mole of interest to me,
That's the kind of mole they use in chemistry.
Chorus: A mole is a unit, or have you heard,
Containing six times ten to the twenty-third,
That's a six with twenty-three zero's at the end,
Much too big a number to comprehend.
Say you had a mole of pennies to distribute 'round the world,
Give to each of the five billion grownups, boys, and girls,
There wouldn't be a single person down and out of luck,
Cause everybody in the world would get a trillion bucks.
Or say you had a mole of paper and stacked it toward the sky,
Paper's awful thin, but that pile would get so high.
It'd reach up into outer space, in fact I think you'd find,
It'd go up to the moon and back, eighty billion times.
Chorus: A mole is a unit, or have you heard,
Containing six times ten to the twenty-third,
That's a six with twenty-three zero's at the end,
Much too big a number to comprehend.
Suppose a mole of marshmallows fell upon the planet,
Over each square inch of land and sea, think that you could stand it?
That layer would be twelve miles high and of course block our sun,
We're talking close to five million trillion tons.
Well, maybe we could save ourselves if we all started eaten',
One marshmallow each second, not two 'cause that'd be cheatin',
With forty five billion people munching, how long do you think it'd take?
Forty million years, and that's without a bathroom break.
Chorus: A mole is a unit, or have you heard,
Containing six times ten to the twenty-third,
That's a six with twenty-three zero's at the end,
Much too big a number to comprehend.
But say you had a mole of atoms, would the pile be immense,
Should I say the answer now or leave you in suspense?
Well, atoms are so very small, very small, you understand,
You could hold a mole of atoms in the palm of your hand.
So shake a little sugar in the middle of your palm,
Now you don't want to spill it, so try and stay calm.
You hardly can imagine and barely realize,
There're more atoms in that sugar than stars up in the sky.
Chorus: A mole is a unit, or have you heard,
Containing six times ten to the twenty-third,
That's a six with twenty-three zero's at the end,
Much too big a number to comprehend.
Today's Class:
Atomic mass unit (amu): a way of measuring the mass of a single atom of an element. To find the amu of an element, look for its atomic mass on the periodic table.
For example, chlorine is 35.5 amu.
To find the amu of an ionic compound, add together the amu of each atom of that compound.
For example, Magnesium Oxide would have an amu of 40.3, because magnesium has an amu of 24.3, and oxygen has an amu of 16.0.
If the compound includes two or more atoms of a single element, add each individual atom's amu to the total amu.
Molecular Mass Formula
1 mole of an element is equal to its amu in grams per mole. For example, one mole of fluoride = 19.0g/mol.
Avogardo's Number is a formula which can calculate how many particles there are in a single mole of any amount of substance.
The formula is: 6.022 x 1023 particles/mol.
Thanks, come again soon!
Thursday, November 11, 2010
Ready for the CELEBRATION yet??
Finally, we are very close to the ending of this chapter which was all about number and value. And now, we are going to have a test on Chapter 3 on MONDAY, NOVEMBER 15th!
Materials will include:
Significant Figures, Measurement & Uncertainty, Scientific Notation, Density, Graphing, Lab 2E, and Unit Conversions! Bring a scientific calculator! Know all formulas for Volume, Area, Density, and Percent Experimental Error.
Let's have a QUICK and EASY review session with this self-quiz!
1. Sig fig include
a)certain digits b)uncertain digits c)all digits d)certain and uncertain digits
2. Which is more precise?
a)2.33 b)2.301 c)2.30 d)2.3
3. Which digit is uncertain in this value: 2.309447
a)7 b) 3 c)0 d)2
4.How many sig fig is in this value: 0.0056930
a)8 b)7 c) 5 d) 4
5. Round 0.90003 to 3 sig figs
a) 0.900 b) 0.90 c) 1.00 d) 0.900030
6. Round 0.34566 to 3 sig figa
a) 0.345 b) 0.340 c) 0.344 d) 0.346
7.Add/Subtract and express the answer in the correct sig fig: 2.40 - 10.293 + 300
a) 292.107 b) 292 c) 290 d)292.11
8.Multiply and express the answer in the correct sig fig: 3.4 x 0.0098
a)0.033 b).0.03 c).0.0 d). 0.03332
9.Calculate Absolute Uncertainty for these 3 values: 2.3, 2.4, 2.5
a) 2.4 +/- 0.1 b) 2.4 +/- 0.2
10. What is the absolute uncertainty of a ruler?
___________________________
11, What is the relative uncertainty if absolute uncertainty is 2 and estimated measurement is 39.2 ?
____________________________
12. Calculate the Mass of a object with volume of 2 and density of 5.
a) 10 b)2.5 c) 0.4
Answer Key:
1. D
2. B
3.A
4.C
5.A
6.D
7.B
8.A
9.A
10. 0.01 cm
11. 5.1%
12. A
Did you get them ALL??? Wasn't it a piece of cake?
well, that didn't cover the whole chapter. I will leave it to your own to study for calculation involved in Lab 2E (Thickness Calculation) and graphing.
GOOD lUCK STUDYING ALL!~



Materials will include:
Significant Figures, Measurement & Uncertainty, Scientific Notation, Density, Graphing, Lab 2E, and Unit Conversions! Bring a scientific calculator! Know all formulas for Volume, Area, Density, and Percent Experimental Error.
Approximately 10 Written and 40 Multiple Choice Questions for a total of about 70 marks!!
1. Sig fig include
a)certain digits b)uncertain digits c)all digits d)certain and uncertain digits
2. Which is more precise?
a)2.33 b)2.301 c)2.30 d)2.3
3. Which digit is uncertain in this value: 2.309447
a)7 b) 3 c)0 d)2
4.How many sig fig is in this value: 0.0056930
a)8 b)7 c) 5 d) 4
5. Round 0.90003 to 3 sig figs
a) 0.900 b) 0.90 c) 1.00 d) 0.900030
6. Round 0.34566 to 3 sig figa
a) 0.345 b) 0.340 c) 0.344 d) 0.346
7.Add/Subtract and express the answer in the correct sig fig: 2.40 - 10.293 + 300
a) 292.107 b) 292 c) 290 d)292.11
8.Multiply and express the answer in the correct sig fig: 3.4 x 0.0098
a)0.033 b).0.03 c).0.0 d). 0.03332
9.Calculate Absolute Uncertainty for these 3 values: 2.3, 2.4, 2.5
a) 2.4 +/- 0.1 b) 2.4 +/- 0.2
10. What is the absolute uncertainty of a ruler?
___________________________
11, What is the relative uncertainty if absolute uncertainty is 2 and estimated measurement is 39.2 ?
____________________________
12. Calculate the Mass of a object with volume of 2 and density of 5.
a) 10 b)2.5 c) 0.4
Answer Key:
1. D
2. B
3.A
4.C
5.A
6.D
7.B
8.A
9.A
10. 0.01 cm
11. 5.1%
12. A
Did you get them ALL??? Wasn't it a piece of cake?
well, that didn't cover the whole chapter. I will leave it to your own to study for calculation involved in Lab 2E (Thickness Calculation) and graphing.
GOOD lUCK STUDYING ALL!~



Friday, November 5, 2010
How was the Quiz??
Hey, everyone~
How did you do on Lab 2E Quiz today??
It was basically a complete summary of out Lab 2E: Determining Aluminum Foil Thickness.
Some of the important formulas were tested:
1. Volume = Length x Width x Height (thickness)
2. Density = Mass / Volume.
3. Mass = Density x Volume
4. Volume = Mass / Density
5. Percentage of Error = (your measurement -accepted measurement) / accepted measurement x 100%
* Also remember the answer should be positive!
I believe these will be important for the Chapter 3 test coming up too!
We also learned graphing skills on EXCEL in the computer lab...
By using EXCEL, we observed the relationship between X and Y more clearly and find the slope and equation. The slope = the Density when X is Volume and Y is Mass.
In the case of the example we did today: Temperature Vs. Density, we had to answer the question why is hot water more dense than cold water?
How did you do on Lab 2E Quiz today??
It was basically a complete summary of out Lab 2E: Determining Aluminum Foil Thickness.
Some of the important formulas were tested:
1. Volume = Length x Width x Height (thickness)
2. Density = Mass / Volume.
3. Mass = Density x Volume
4. Volume = Mass / Density
5. Percentage of Error = (your measurement -accepted measurement) / accepted measurement x 100%
* Also remember the answer should be positive!
I believe these will be important for the Chapter 3 test coming up too!
We also learned graphing skills on EXCEL in the computer lab...
By using EXCEL, we observed the relationship between X and Y more clearly and find the slope and equation. The slope = the Density when X is Volume and Y is Mass.
In the case of the example we did today: Temperature Vs. Density, we had to answer the question why is hot water more dense than cold water?
Wednesday, November 3, 2010
November 3rd
Well, hi there,,,,
this is my first time to type in this blog,, so i am kind of nervous?!
Okay,, back to the point.
For the chemistry lesson today,,
we did an experiment from the lab book, it is the 2E lab-- DETERMINING ALUMINIUM FOIL THICKNESS.
Well,,, apparantly,, it is not a hard experiement to do.
first, find three aluminium foil and measures the width and length, then weight it.
after that,, just use the formulas provided to get the answer for it
#### IMPORTANT FORMULAS####
1) Volume of a rectangular solid V=LWH (length x width x height)
2) Density of a substance D=m/V (mass/volume)
Well,, i guess that's the end for my first time?!
P.S remember that we have a quiz of the lab 2E on this FRIDAY!!! Good luck~~ =]
this is my first time to type in this blog,, so i am kind of nervous?!
Okay,, back to the point.
For the chemistry lesson today,,
we did an experiment from the lab book, it is the 2E lab-- DETERMINING ALUMINIUM FOIL THICKNESS.
Well,,, apparantly,, it is not a hard experiement to do.
first, find three aluminium foil and measures the width and length, then weight it.
after that,, just use the formulas provided to get the answer for it
#### IMPORTANT FORMULAS####
1) Volume of a rectangular solid V=LWH (length x width x height)
2) Density of a substance D=m/V (mass/volume)
Well,, i guess that's the end for my first time?!
P.S remember that we have a quiz of the lab 2E on this FRIDAY!!! Good luck~~ =]
Tuesday, November 2, 2010
November 1, 2010
Today is Monday, November 1 (can u believe that it is already November?!?!?!) and we took our quiz and started learning on our new subject: DENSITY !!!! yay !!!
Here is what we did in order:
- First we corrected our review sheet for sig figs.
- Then we took our quiz
(which was on Significent Figures, Uncertainty, Scientific Notation and Measurement)
- And for the remainder of the class we took notes on Density and we got a worksheet for homework.
* Here is a quick lesson on Density:
Facts;
- Density = mass divided by volume
- Volume = mass divided by density
- Mass = density times volume
- Density of a solid = g/cm cubed
liquid = g/mL
water = 1.0 g/mL or 1000 g/L
- If the density of an object is greater than the density of liquid the object will sink
And if the density of an object is less than the density of liquid the object will float
Examples of Density Problems (see if you can figure them out)
1) Calculate the density in g/mL of aluminum if a 50 mL block weighs135 g.
2) Calculate the mass in a 200 cc block of Titanium with a density of 4.51 g. per cc.
3) Calculate the mass of a liquid with a density of 3.2 g/mL and a volume of 25 mL.
4) An irregular object with a mass of 18 kg displaces 2.5 L of water when placed in a large overflow container. Calculate the density of the object.
5) A graduated cylinder has a mass of 80 g when empty. When 20 mL of water is added, the graduated cylinder has a mass of 100 g. If a stone is added to the graduated cylinder, the water level rises to 45 mL and the total mass is now 156 g. What is the density of the stone?
Since density is known worldwide and can be connected to so many things here are some news articles and papers that you should check out!
1. http://www.warsawvoice.pl/WVpage/pages/article.php/22750/article
2.http://news.softpedia.com/news/Saturn-s-Rings-Are-Proxies-for-Galaxies-164138.shtml
3. http://www.usnews.com/science/articles/2010/11/01/revealing-the-galaxys-dark-side.html
Here is a website that focuses all on science news and very interesting news:
1. http://insciences.org/articles.php?tag=Chemistry
Images to help with density:
A density joke that ought to make you chuckle!
- In world war 1 a great big british mine found itself sinking through the ocean right next to a great big german mine. After a while the great big british mine said - 'I say, we seem to be sinking at exactly the same rate - we must have the same density!'
'Somehow I doubt zis!' replied the great big german mine; 'It is just zat great mines sink alike...'
And four videos about Density & Volume & Mass!
- http://www.youtube.com/watch?v=fqLCwuKMBMA
- http://www.youtube.com/watch?v=rxb_6UANXqU
- http://www.youtube.com/watch?v=grWG_U4sgS8&feature=related
- http://www.youtube.com/watch?v=uvy4nWh0KwE&feature=related
Last but not least for next class....................
- We are doing Lab 2E. So dont forget to make a chart of the procedures and make a copy of Table 1.
- Finish the density problem worksheet.
Here is what we did in order:
- First we corrected our review sheet for sig figs.
- Then we took our quiz
(which was on Significent Figures, Uncertainty, Scientific Notation and Measurement)
- And for the remainder of the class we took notes on Density and we got a worksheet for homework.
* Here is a quick lesson on Density:
Facts;
- Density = mass divided by volume
- Volume = mass divided by density
- Mass = density times volume
- Density of a solid = g/cm cubed
liquid = g/mL
water = 1.0 g/mL or 1000 g/L
- If the density of an object is greater than the density of liquid the object will sink
And if the density of an object is less than the density of liquid the object will float
Examples of Density Problems (see if you can figure them out)
1) Calculate the density in g/mL of aluminum if a 50 mL block weighs135 g.
2) Calculate the mass in a 200 cc block of Titanium with a density of 4.51 g. per cc.
3) Calculate the mass of a liquid with a density of 3.2 g/mL and a volume of 25 mL.
4) An irregular object with a mass of 18 kg displaces 2.5 L of water when placed in a large overflow container. Calculate the density of the object.
5) A graduated cylinder has a mass of 80 g when empty. When 20 mL of water is added, the graduated cylinder has a mass of 100 g. If a stone is added to the graduated cylinder, the water level rises to 45 mL and the total mass is now 156 g. What is the density of the stone?
Since density is known worldwide and can be connected to so many things here are some news articles and papers that you should check out!
1. http://www.warsawvoice.pl/WVpage/pages/article.php/22750/article
2.http://news.softpedia.com/news/Saturn-s-Rings-Are-Proxies-for-Galaxies-164138.shtml
3. http://www.usnews.com/science/articles/2010/11/01/revealing-the-galaxys-dark-side.html
Here is a website that focuses all on science news and very interesting news:
1. http://insciences.org/articles.php?tag=Chemistry
Images to help with density:
A density joke that ought to make you chuckle!
- In world war 1 a great big british mine found itself sinking through the ocean right next to a great big german mine. After a while the great big british mine said - 'I say, we seem to be sinking at exactly the same rate - we must have the same density!'
'Somehow I doubt zis!' replied the great big german mine; 'It is just zat great mines sink alike...'
And four videos about Density & Volume & Mass!
- http://www.youtube.com/watch?v=fqLCwuKMBMA
- http://www.youtube.com/watch?v=rxb_6UANXqU
- http://www.youtube.com/watch?v=grWG_U4sgS8&feature=related
- http://www.youtube.com/watch?v=uvy4nWh0KwE&feature=related
Last but not least for next class....................
- We are doing Lab 2E. So dont forget to make a chart of the procedures and make a copy of Table 1.
- Finish the density problem worksheet.
Sunday, October 31, 2010
October 28
There are new notes on Sigfigs today!
Topic for today are Accuracy and Precision.
Precision is how reproducible a measurement is compared to other similiar measurements.
Accuracy is how close the measument (or average measuremen) comes to the accepted or real value.
Measurement and Uncertainty
-no measurement is exact.
-every measurement is best estimate
-except if we can count the # of objects
Absolute Uncertainty
-Uncertainty expressed in the units of measurement (not as ratio)
-Method 1: make at least 3 measurements and calculate the average. Absolute unit is the largest difference between average and lowest/highest reasonable measurement. (ie. discard unreasonable data 1st!!)
-Method 2: determine the uncertainty of each instrument. Measure to the best precision when making a measurement= estimate to a fraction 0.1 of the smallest segment on instrumental scale.
EX. ruler has smallest division of 1mm, so best precision should be break into 10 equal pieces over 1mm.
Relative Uncertainty and Sigfigs!
1). Relative Uncertainty = absolute uncertainty / estimated measurement.
can be expressed in percent %
EX. 0.05 cm +/- 0.01
(0.01/ 0.05) (100%) = 20% relative uncertainty.
2). or use sigfigs.
We did the Equipment Measuring Activity sheet together. With each measurement, we recorded the digits until the uncertain digit and state # of sig figs as well as Acceptable measurement which we will cover next class.

HOMEWORK:
sig figs and accuracy and precision worksheet, Sig fig quiz on monday!
Topic for today are Accuracy and Precision.
Precision is how reproducible a measurement is compared to other similiar measurements.
Accuracy is how close the measument (or average measuremen) comes to the accepted or real value.
Measurement and Uncertainty
-no measurement is exact.
-every measurement is best estimate
-except if we can count the # of objects
Absolute Uncertainty
-Uncertainty expressed in the units of measurement (not as ratio)
-Method 1: make at least 3 measurements and calculate the average. Absolute unit is the largest difference between average and lowest/highest reasonable measurement. (ie. discard unreasonable data 1st!!)
-Method 2: determine the uncertainty of each instrument. Measure to the best precision when making a measurement= estimate to a fraction 0.1 of the smallest segment on instrumental scale.
EX. ruler has smallest division of 1mm, so best precision should be break into 10 equal pieces over 1mm.
Relative Uncertainty and Sigfigs!
1). Relative Uncertainty = absolute uncertainty / estimated measurement.
can be expressed in percent %
EX. 0.05 cm +/- 0.01
(0.01/ 0.05) (100%) = 20% relative uncertainty.
2). or use sigfigs.
We did the Equipment Measuring Activity sheet together. With each measurement, we recorded the digits until the uncertain digit and state # of sig figs as well as Acceptable measurement which we will cover next class.

HOMEWORK:
sig figs and accuracy and precision worksheet, Sig fig quiz on monday!
Tuesday, October 26, 2010
October 26th
Significant Digits
What are significant digits?
For example, 439 has 3 significant digits - 4, 3, and 9.
98963.4 has 6 significant digits.
11 has 2 significant digits, and so on and so forth.
Rounding
Give me four different numbers.
Okay...
um...
18.36...
18.32...
18.357...
annnnnd...18.35.
Couldn't have picked them better myself. Okay! Now we'll explore the four different rules of rounding, using these numbers!
What do you think we should round to this time, mmm?
To the tenths place would be nice.
To the tenth it is, then.
To round, first look at the number that's one to the right of the place you're rounding to. In this case, we look at the digit in the hundredth position.
Didn't I do this in primary school?
Yes. Shut up.
Adding and subtracting is so simple it's ridiculous. Let's take a look at some examples and you can off on your merry way.
72.456 mL
+ 87.222 mL
^ Here we have a simple addition problem. Do what you would normally do to solve for the answer.
Multiplying and Dividing
Same goes for multiplying and dividing too. First, do the problem as you normally would, then round the answer to the fewest number of significant digits.
8.82 km x 4.1 km = 36.162 km ²
36.162 rounded to 2 significant figures ---> 36 km ²
Homework:
The two significant figures worksheets :)
Extras:
^ Originally had a neat music video for you, but right after publishing the post, it was discovered that the video wouldn't play. Boo.
So here's a tutorial on sig. figs instead. Skip to around 1:50-ish for the actual explanation; everything before that is just the guy drifting in his own little world.
Significant Digits
^ Quite a useful website. Has questions that you can do, with instant answers.
What are significant digits?
Significant digits are, well, basically any number really. The number of significant digits that a certain value has is almost always equal to how many numbers there are in the value.
For example, 439 has 3 significant digits - 4, 3, and 9.
98963.4 has 6 significant digits.
11 has 2 significant digits, and so on and so forth.
Okay, are we done here?
 |
| Well done, smarty. |
Not so fast. First of all, realize that significant digits include only the certain digits in a value, and only the first uncertain digit.
Huh?
Let's take a value like 3.27 for example. We know that the 3 and the 2 are concrete digits that we are absolutely sure of. The 7, on the other hand, is a bit iffy. It could easily have been a product of rounding up (3.269) or rounding down (3.274). The significant numbers would include all of the certain digits, and just the first uncertain digit. |
| Only after the 7 do the digits qualify as "significant digits". |
Secondly, notice how the above values only contain non-zero numbers. The situation gets more complicated when zeros are thrown into the mix.
- Leading zeros do not qualify as significant digits: so for a number like 0.0045, there are only 2 sig. figs.
- Zeros that come after a significant digit after a decimal point qualify as significant digits: 0.500 has 3 sig. figs.
- Zeros that come after a significant digit, but before a decimal point do not qualify as significant digits: 8000 only has 1 sig. fig.
Rounding
Give me four different numbers.
Okay...
um...
18.36...
18.32...
18.357...
annnnnd...18.35.
Couldn't have picked them better myself. Okay! Now we'll explore the four different rules of rounding, using these numbers!
What do you think we should round to this time, mmm?
To the tenths place would be nice.
To the tenth it is, then.
To round, first look at the number that's one to the right of the place you're rounding to. In this case, we look at the digit in the hundredth position.
- Let's take the first number, 18.36. In this case, the hundredth position is occupied by 6. 6 is bigger than 5. If the number in the position that's one to the right of the place you're rounding to is bigger than 5, you round up. 18.36 ---> 18.4
- Let's take the second number, 18.32. In this case, the hundredth position is occupied by 2. 2 is smaller than 5. If the number in the position that's one to the right of the place you're rounding to is smaller than 5, you keep the number the same. 18.32 ---> 18.3
- Let's take the third number, 18.357. In this case, the hundredth position is occupied by 5. In an event like this, look to see if there are any more digits to the right of 5. If there are, you would round up. 18.357 ---> 18.4
- Let's take the first number, 18.35. Now there are no numbers after the 5. In an event like this, you would round to make the last digit an even digit. 18.35 ---> 18.4
Didn't I do this in primary school?
Yes. Shut up.
Adding and subtracting is so simple it's ridiculous. Let's take a look at some examples and you can off on your merry way.
72.456 mL
+ 87.222 mL
^ Here we have a simple addition problem. Do what you would normally do to solve for the answer.
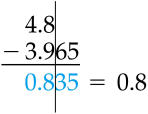 |
| Leave answers with the fewest decimal places possible. |
72.456 mL
+ 87.222 mL
159.678 mL
Now, round using the previously shown method, leaving only one decimal place.
159.678 ---> 159.7 (because 7 > 5)
Multiplying and Dividing
Same goes for multiplying and dividing too. First, do the problem as you normally would, then round the answer to the fewest number of significant digits.
8.82 km x 4.1 km = 36.162 km ²
36.162 rounded to 2 significant figures ---> 36 km ²
Homework:
The two significant figures worksheets :)
Extras:
^ Originally had a neat music video for you, but right after publishing the post, it was discovered that the video wouldn't play. Boo.
So here's a tutorial on sig. figs instead. Skip to around 1:50-ish for the actual explanation; everything before that is just the guy drifting in his own little world.
Significant Digits
^ Quite a useful website. Has questions that you can do, with instant answers.
Wednesday, October 20, 2010
October 19
Today we did lab 3B: Separation of a Mixture by Paper Chromatography
The purpose of this lab is to assemble and operate a paper chromatography apparatus, to study the meaning and significance of Rf values, and to identify the components of mixtures by means od Rf values.
For Part I, we set up the paper chromatography and prepare for the experiment. For Part II, we started with the blue dye on the chromatography paper. After 20 minutes, we are able to identify the solvent front and solute front and measure their distance to determine the Rf value. Part III is a similar process with two different dyes, one green and one unknown. Our task is to identify the components of the mixtures. The class as a whole recorded Class Results for Rf values as a reference. Finally, for table 3, we did the comparison for components of the coloring. The result for the green dye was yellow and blue by obervation and using Rf values. The result for the unknown dye (brown) was yello, red and blue.
At the end of our lab, we had time to do our lab report. As for conclusion, answer the question how to identify the components of a mixture. We came up with the answer to find Rf value first and compare with the standard value to match up the correct component.
Here is a link demonstrating similar concept of Rf value and the experiment of paper chromatograhy!
http://www.youtube.com/watch?v=3ZP_E0eTmMU
Our homework
The purpose of this lab is to assemble and operate a paper chromatography apparatus, to study the meaning and significance of Rf values, and to identify the components of mixtures by means od Rf values.
For Part I, we set up the paper chromatography and prepare for the experiment. For Part II, we started with the blue dye on the chromatography paper. After 20 minutes, we are able to identify the solvent front and solute front and measure their distance to determine the Rf value. Part III is a similar process with two different dyes, one green and one unknown. Our task is to identify the components of the mixtures. The class as a whole recorded Class Results for Rf values as a reference. Finally, for table 3, we did the comparison for components of the coloring. The result for the green dye was yellow and blue by obervation and using Rf values. The result for the unknown dye (brown) was yello, red and blue.
At the end of our lab, we had time to do our lab report. As for conclusion, answer the question how to identify the components of a mixture. We came up with the answer to find Rf value first and compare with the standard value to match up the correct component.
Here is a link demonstrating similar concept of Rf value and the experiment of paper chromatograhy!
http://www.youtube.com/watch?v=3ZP_E0eTmMU
Our homework
- finish 3B lab report
- review answers online
- Chapter1 and 2 test next class (everything we've done so far, except safety)
- Bring scientific calculator
Sunday, October 17, 2010
October 15, 2010
* Today in class we first took quite a bit of notes, then we got a review sheet to complete and last but not least we started working on our lab that we will do next class (Tuesday).

* Those pictures/diagrams should help you alot along with the notes that u already have. Now here are three videos that also go along with seperation techniques so now unfortunately you will have no excuse to not knowing the material:
1. http://www.youtube.com/watch?v=7VrOQg6kFow
2. http://www.youtube.com/watch?v=QEex788j-yk
3. http://www.youtube.com/watch?v=vcwfhDhLiQU&feature=related
* Now secondly, a reminder that you will have to finish the review sheet by next class (Tuesday) both sides but skip #7 !!!!
* I will first sum up the notes that we took:
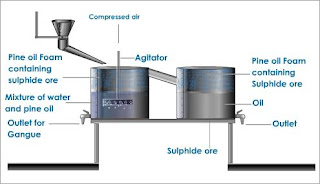
- We were introduced to Separation Techniques. There are many basic techniques that include: Filtration, Floatation, Crystallization & Extraction, Pistillation, Chromatography, Hand Separation & Evaporation, Gravity Separation, Solvent Extraction, and Distillation. Here are some pictures & diagrams to explain all of them in a simple way;
Distillation (left and underneath) Floatation (right and underneath)
Filtration (left and underneath) Crystallization & Extraction (right and underneath)
Pistillation (left and underneath) Chromatography (right and underneath)
Solvent Extraction (left and underneath) Evaporation (right and underneath)

* Those pictures/diagrams should help you alot along with the notes that u already have. Now here are three videos that also go along with seperation techniques so now unfortunately you will have no excuse to not knowing the material:
1. http://www.youtube.com/watch?v=7VrOQg6kFow
2. http://www.youtube.com/watch?v=QEex788j-yk
3. http://www.youtube.com/watch?v=vcwfhDhLiQU&feature=related
* Now secondly, a reminder that you will have to finish the review sheet by next class (Tuesday) both sides but skip #7 !!!!
* Thirdly, next class we are doing a lab; expirement 3B - seperation of a mixture by paper chromatography. Remember to bring your lab textbook and to create and bring a flowchart of the procedure of the lab. And there are three tables to copy from the lab. Here is an example of what the flowchart should look like:
* Thank You and I Hope You Had A Great Weekend!!!!
Subscribe to:
Comments (Atom)